Eli Lilly investigators estimate that the company's experimental Alzheimer's disease drug donanemab could clear amyloid plaque brain deposits in patients for up to four years, according to a study published September 12 in JAMA Neurology.
The researchers developed a statistical model based on PET scans to calculate potential clinical outcomes for patients who responded well to donanemab during a previous 18-month, phase II clinical trial.
"The exposure-response model of amyloid plaque level over time suggested that it might take 3.9 years for plaques to reaccumulate," wrote corresponding author Dr. John Sims, a senior medical director at Eli Lilly.
In addition, the early clearance of amyloid plaque in patients shown in the trial was associated with a slowing of tau accumulation, which is triggered by amyloid plaque and considered to be the main cause of Alzheimer's disease dementia, the authors noted.
Amyloid deposits and tau protein neurofibrillary tangles are the two major pathological hallmarks of Alzheimer's disease. The accumulation of these proteins is a continuous process that starts decades before the onset of symptoms.
Donanemab is a monoclonal antibody designed to bind to beta-amyloid protein that already has formed into plaques. The drug then stimulates the immune system to attack these plaque deposits and clear them from the brain.
In January 2021, Eli Lilly published the results of its TRAILBLAZER-ALZ phase II clinical trial to test the efficacy and safety of donanemab. In contrast to patients on placebo, a majority of patients treated with the drug experienced a robust amyloid plaque reduction at 24 weeks, according to the authors.
The primary focus of the phase II trial was the use of the "Centiloid (CL) scale," a method developed to standardize the results of amyloid PET scans in measurable units, with a score of 100 indicating "typical" Alzheimer's disease. In the trial, patients were considered to be completely clear with an amyloid plaque level of 24.1 CL, and some patients reached less than 11 CL by 24 weeks, the authors wrote.
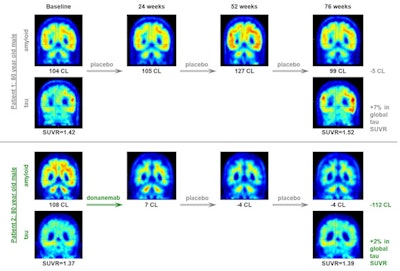 Representative amyloid and tau PET scans showing changes after placebo and donanemab treatment over the course of 76 weeks. Tau level is measured using an Alzheimer's disease-signature weighted neocortical SUVR (standardized uptake value ratio) with respect to cerebellum gray as a reference. Image courtesy of JAMA Neurology.
Representative amyloid and tau PET scans showing changes after placebo and donanemab treatment over the course of 76 weeks. Tau level is measured using an Alzheimer's disease-signature weighted neocortical SUVR (standardized uptake value ratio) with respect to cerebellum gray as a reference. Image courtesy of JAMA Neurology.In the present study, the group developed an exposure-response model that incorporated all available longitudinal pharmacokinetic and PET data from participants in the trial. According to the model projections, patients who achieved an amyloid load of 11 CL or less at week 24 and then discontinued amyloid treatment, the median time to reaccumulate amyloid from 11 CL to the 24.1 CL threshold could be 3.9 years, the authors wrote.
In addition, analysis of flortaucipir F-18 PET scans acquired of responding patients after 72 weeks showed a statistically significant 34% slowing of overall tau levels compared to placebo, according to the findings.
"This reduced progression strengthens the hypothesis of an amyloid-mediated tauopathy and has important implications for anti-amyloid treatments because it has previously been shown that the amount of brain tau pathology predicts subsequent cognitive decline," the authors wrote.
The researchers noted limitations, namely that these results are from exploratory, post hoc analyses, which carry a risk for bias and warrant cautious interpretations. However, new trials and planned analyses will be able to ascertain whether these findings are robust, they added.
Ultimately, the "parsimonious interpretation" of the study is that early reduction of amyloid plaque reduces the amount of time during which amyloid plaque can drive downstream tau pathology, and thus possibly prevent disease progression, the authors wrote.
"These results underscore the importance of amyloid plaques in [Alzheimer's disease] and understanding amyloid status for treatment decisions," Sims and colleagues concluded.
Disclosure notice: This study was funded by Eli Lilly, and all co-authors are employees of Eli Lilly.





















