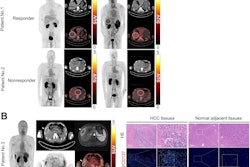
Whole-body PET imaging can help clinicians visualize how patients may respond to immune checkpoint inhibitor therapy, according to a study published December 5 in Nature Medicine.
In a first trial in patients, a group led by Dr. Elisabeth de Vries, PhD, of the University of Groningen in the Netherlands evaluated the use of a zirconium-89 (Zr-89)-based radiotracer that reveals the activity of CD8 T cells triggered to kill cancer cells in patients undergoing immune checkpoint inhibitor (ICI) therapy.
"We demonstrated that CD8-positive T cell presence in tumor lesions imaged before ICI could be predictive for [overall survival], highlighting the potential of CD8 imaging as a predictive biomarker to personalize treatment for patients," the group wrote.
Immune checkpoint inhibitors (ICIs) boost CD8-positive T-cell-mediated immunity and thus harness patients' immune system to kill cancer cells. The approach has revolutionized cancer therapy, yet systemic CD8-positive T-cell distribution, a potential biomarker of ICI response, remains poorly characterized, according to the authors.
In this study, the team aimed first to determine the safety of the approach using a tracer they developed named 89-ZED88082A and then analyzed whether it could reveal individual patient response to ICI therapy.
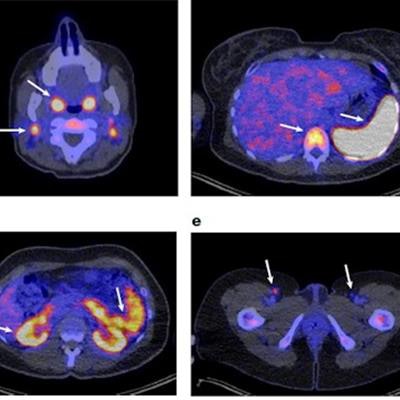
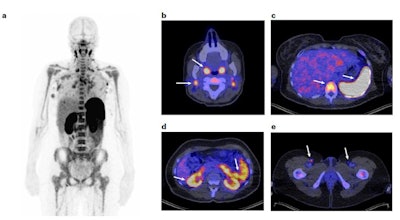 (a) Representative 89-ZED88082A-PET scan maximum intensity projection image on day two; (b-e), Axial views of the same scan fused with low-dose CT. Arrows indicate uptake in Waldeyer's ring, cervical lymph nodes (b), spleen, bone marrow (c), renal cortex, small intestine (d) and inguinal lymph nodes (e). Image courtesy of Nature Medicine.
(a) Representative 89-ZED88082A-PET scan maximum intensity projection image on day two; (b-e), Axial views of the same scan fused with low-dose CT. Arrows indicate uptake in Waldeyer's ring, cervical lymph nodes (b), spleen, bone marrow (c), renal cortex, small intestine (d) and inguinal lymph nodes (e). Image courtesy of Nature Medicine.Between February 2019 and November 2020, the researchers recruited 39 cancer patients with solid tumors of various cancer types and performed 89-ZED88082A-PET imaging before and 30 days after the patients started ICI therapy, namely with atezolizumab and/or ipilimumab.
No 89-ZED88082A-related side effects occurred, with adverse events due to ICI consistent with reports from previous studies. The researchers found that a 10 mg tracer dose and PET scanning on day two to be optimal.
Further analysis showed that uptake of the tracer by tumors measured by maximum standardized uptake value (SUVmax) was associated with longer overall patient survival. Patients with a baseline mean SUVmax of more 5.2 showed a trend towards superior progression-free survival and superior overall survival compared to patients with an uptake below this level.
Ultimately, in the short term, the trial's significance is that it also showed a large spatial and temporal variation in the presence of CD8-positive T cells, not only in patients who responded to therapy but also within individual patients and between lesions. This means that CD8-positive T cells are important players in tumor destruction by ICI but that their activation is not as clear cut as previously thought, they wrote.
"For future studies, we envision also an earlier second imaging time point, namely within two weeks after starting ICI therapy, to capture pharmacodynamic changes before substantial tumor shrinkage," the group concluded.