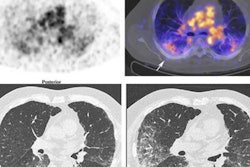
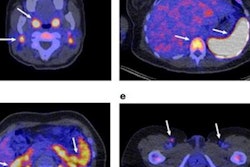
A new PET radiotracer has the potential for visualizing responses in liver cancer patients undergoing immunotherapy, according to a study published December 12 in the Journal of Nuclear Medicine.
In a translational study, researchers synthesized the imaging agent, called F-18 AlF-NOTA-BCP137, and then tested it in mice and humans for the first time. The findings merit further validation in larger clinical trials, noted lead author Kai Cheng, MD, of the Shandong First Medical University in Jinan, China, and colleagues.
“Our findings revealed the potential of this imaging method for the early noninvasive evaluation of activated T cells and tumor responses to immunotherapy,” the group wrote.
Immune checkpoint inhibitors -- drugs that help the immune system attack tumors -- have revolutionized cancer therapy, yet variability in patient responses call for predictive biomarkers to optimize treatment outcomes, the authors explained. One such promising biomarker is CD137, a receptor expressed on activated T cells, they noted.
Thus, in this study, the group synthesized F-18 AlF-NOTA-BCP137 and first evaluated it using micro-PET/CT imaging in mice with tumors. The findings from these animal studies indicated that uptake of the tracer predicted early therapeutic responses to immunotherapies and was positively associated with increased survival rates.
Next, the researchers enrolled five patients diagnosed with hepatocellular carcinoma, all of whom underwent whole-body PET/CT imaging approximately 50 minutes after injection of F-18 AlF-NOTA-BCP137. The researchers defined a positive imaging outcome as a tumor-to-muscle maximum standardized uptake value (SUVmax) ratio greater than 3 to stratify patients into immunotherapy responders and nonresponders.
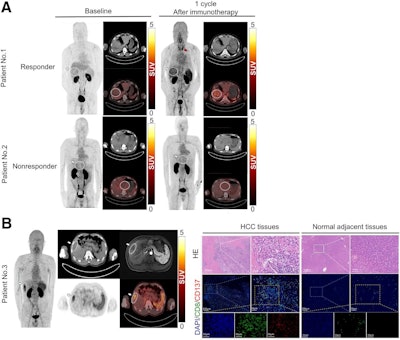 PET/CT images of F-18 AlF-NOTA-BCP137 in patients with hepatocellular carcinoma (HCC) and immunofluorescence analysis of patient tissues. (A) F-18 AlF-NOTA-BCP137 PET/CT images before and after immunotherapy for patient 1 and patient 2. (B) F-18 AlF-NOTA-BCP137 PET/CT and MR images of patient 3 (left panels) after one cycle of immunotherapy. PET images showed higher uptake by tumor and spleen, and PET result was evaluated as positive; therefore, the patient was considered to be responder to treatment and underwent surgical resection. Representative images of hematoxylin and eosin (HE) staining and multiplex immunofluorescence of CD8 and CD137 (right panels) in HCC tumor and adjacent normal tissues of patient 3 after surgery. Nuclei were stained with 4′,6-diamidino-2-phenylindole. Primary tumors are indicated by white arrows, and increased thyroid uptake is indicated by red arrow in PET images. Image courtesy of the Journal of Nuclear Medicine.
PET/CT images of F-18 AlF-NOTA-BCP137 in patients with hepatocellular carcinoma (HCC) and immunofluorescence analysis of patient tissues. (A) F-18 AlF-NOTA-BCP137 PET/CT images before and after immunotherapy for patient 1 and patient 2. (B) F-18 AlF-NOTA-BCP137 PET/CT and MR images of patient 3 (left panels) after one cycle of immunotherapy. PET images showed higher uptake by tumor and spleen, and PET result was evaluated as positive; therefore, the patient was considered to be responder to treatment and underwent surgical resection. Representative images of hematoxylin and eosin (HE) staining and multiplex immunofluorescence of CD8 and CD137 (right panels) in HCC tumor and adjacent normal tissues of patient 3 after surgery. Nuclei were stained with 4′,6-diamidino-2-phenylindole. Primary tumors are indicated by white arrows, and increased thyroid uptake is indicated by red arrow in PET images. Image courtesy of the Journal of Nuclear Medicine.
According to this criteria, two patients had positive CD137 PET/CT scans, with one patient showing stable disease and the other showing a partial treatment response. The remaining three patients had negative PET/CT results and were assessed as having progressive disease (nonresponders), which suggests that positive CD137 PET/CT scans are associated with better treatment outcomes, the group wrote.
In addition, the patient who showed a partial response to treatment underwent surgical resection of the tumor on the second day after the PET/CT imaging, which allowed the researchers to collect tissue samples for further study. This analysis showed more CD8+ T cells and higher CD137 expression in the tumor, indicating effective T cell activation from therapy. In contrast, the reduced presence of CD8+ T cells and CD137 in adjacent normal tissue highlighted the tracer’s specificity, the group wrote.
Ultimately, this was a preliminary clinical study involving a small number of patients, and while the results are promising, a larger patient cohort is needed to validate F-18 AlF-NOTA-BCP137’s predictive value and its correlation with patient outcomes, the researchers noted.
“We demonstrated the utility of F-18 AlF-NOTA-BCP137 PET imaging in the assessment of CD137 expression, and our findings revealed the potential of this imaging method for the early noninvasive evaluation of activated T cells and tumor responses to immunotherapy,” the group concluded.
The full study is available here.