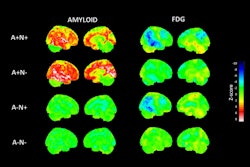
PET scans have revealed specific brain pathology that may drive sleep disturbances in Alzheimer’s disease patients, according to a study published February 8 in the Journal of the Neurological Sciences.
In a large group, abnormal nighttime behaviors were linked with greater baseline tau on F-18 flortaucipir PET scans, with the finding offering a potential new target for delaying cognitive decline, noted lead author Ryan Kim, of Harvard University in Cambridge, MA, and colleagues.
“Our results help identify high-risk individuals who could benefit from sleep-related interventions aimed to delay cognitive decline and [Alzheimer's disease],” the group wrote.
Alzheimer's disease is commonly associated with impaired sleep, although the underlying mechanisms for this remain unclear, the authors explained. Two recent studies have linked sleep disturbance with regional tau on brain PET scans, but only in small groups of patients, they added.
To explore the association further, the researchers first analyzed PET imaging in 254 Alzheimer’s disease patients who had participated in a previous trial. Of these, 119 (43%) had normal cognition, 132 (24%) had mild cognitive impairment, and three (2%) had Alzheimer’s disease. Patients also had follow-up scans, with a mean follow-up duration between the two of 2.8 years.
Next, the researchers assessed impaired sleep in the patients in two ways: by documentation of sleep disturbances in their medical history and by abnormal nighttime behaviors reported on a Neuropsychiatric Inventory Questionnaire (NPIQ).
According to an analysis, a medical history of sleep impairment was associated with greater baseline tau in the metatemporal, Braak 1, and Braak 4 regions – brain regions associated with memory and cognition, the group wrote.
Separated by group, a history of sleep disturbances was associated with greater tau in the meta-temporal and Braak 4 regions in the cognitively normal group and greater tau in the Braak 1 region in the mildly cognitively impaired group, they noted.
Abnormal nighttime behaviors were also associated with greater baseline tau in the meta-temporal region and greater cognitive impairment in all patients over the study period, they added.
“Sleep impairment was associated with tauopathy and cognitive decline,” the group wrote.
Ultimately, there could be several potential “bidirectional” mechanisms to explain the underlying pathophysiology linking sleep and tau, the authors noted.
For instance, insufficient or impaired sleep could lead to neuronal injury and higher tau levels, or conversely, higher tau on F-18 flortaucipir PET scans could reflect more tau pathology in the sleep centers of the brain, they suggested.
Nonetheless, the results have several implications for clinical practice, they wrote.
“This finding again presents an opportunity for intervention, as identification of abnormal nighttime behaviors on the NPIQ could identify individuals who could benefit from disease-modifying therapy,” the researchers concluded.
The full study is available here.