F-18 fluroestradiol (FES)-PET/CT appears as effective as standard imaging for staging women with estrogen receptor (ER)-positive locally advanced breast cancer or evaluating suspected recurrence, according to a study published July 26 in JAMA Open Network.
In a clinical trial conducted at the Hoag Family Cancer Institute in Irvine, CA, researchers tested the technique versus CT bone scans or FDG-PET/CT and found that it detected an equivalent number of cases.
“These findings suggest that FES-PET/CT could be considered for staging or detection of recurrence in patients with ER-positive breast cancer,” noted lead author Gary A. Ulaner, MD, PhD, of the University of Southern California, Los Angeles.
Women with locally advanced breast cancer (LABC) have a high risk for distant metastases at initial diagnosis and undergo systemic staging with imaging to help determine whether patients will undergo therapy and what therapies are chosen.
F-18 fluoroestradiol (Cerianna, GE HealthCare) is a PET radiotracer approved in the U.S. in 2020 that targets estrogen receptors, which are expressed in about 70% of breast cancers. Researchers are conducting ongoing research to define the clinical scenarios where the tracer may be most helpful.
To date, there are no data comparing FES-PET with current standard-of-care imaging, specifically CT bone scans or FDG PET/CT, for staging ER-positive LABC or evaluating suspected recurrences, the authors noted.
In this phase II study, the researchers enrolled 124 patients and split them into two cohorts: 62 patients with ER-positive LABC (cohort 1) and 62 patients with suspected recurrence (cohort 2). Participants underwent both standard-of-care imaging and experimental FES PET/CT within 14 days of each other. When there were suspicious lesions on imaging, one was biopsied for histopathological reference standard to confirm presence (true positive) or absence (false positive) of lesions.
According to the results, out of 14 metastases detected in cohort 1, standard-of-care imaging detected 12 and FES detected 11 (p > 0.99). In cohort 2, out of 23 true-positive findings, SOC detected 16 and FES detected 18 (p = 0.77). In addition, there were 30 patients with invasive lobular carcinoma (ILC), and 11 of these patients had biopsy-proven distant metastases or recurrent malignant tumors, with six detected by FES PET/CT only.
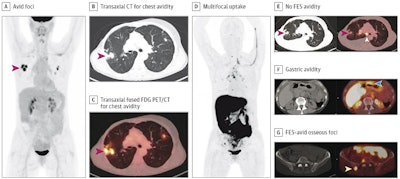 The patient was a woman in her 50s with suspected recurrence of invasive lobular breast cancer. A, maximum intensity projection image from FDG-PET/CT demonstrating avid foci in the chest (red arrow). Transaxial CT (B) and transaxial fused FDG-PET/CT image (C) showing that the chest avidity corresponds to FDG-avid lung nodules (red arrows) suspicious for malignancy. The lung nodule was subsequently biopsied but found to represent benign granulomatous inflammation and thus a false positive on FDG-PET/CT. D, maximum intensity projection image from FES-PET/CT demonstrating multifocal uptake suspicious for malignancy. E, transaxial CT and fused FES-PET/CT demonstrating no FES-avidity in the biopsy proven benign granulomatous lung nodules (red arrows), thus true negative on FES-PET/CT. FES-avidity in nodes (white arrow) is suspicious for malignancy. F, transaxial CT and fused FES-PET/CT demonstrates gastric avidity (blue arrow) suspicious for malignancy. G, transaxial CT and fused FES PET/CT demonstrates FES-avid osseous foci (yellow arrow) suspicious for malignancy. This osseous focus was subsequently biopsied and proved to be an osseous metastasis and thus true positive on FES-PET/CT.Image courtesy of JAMA Open Network
The patient was a woman in her 50s with suspected recurrence of invasive lobular breast cancer. A, maximum intensity projection image from FDG-PET/CT demonstrating avid foci in the chest (red arrow). Transaxial CT (B) and transaxial fused FDG-PET/CT image (C) showing that the chest avidity corresponds to FDG-avid lung nodules (red arrows) suspicious for malignancy. The lung nodule was subsequently biopsied but found to represent benign granulomatous inflammation and thus a false positive on FDG-PET/CT. D, maximum intensity projection image from FES-PET/CT demonstrating multifocal uptake suspicious for malignancy. E, transaxial CT and fused FES-PET/CT demonstrating no FES-avidity in the biopsy proven benign granulomatous lung nodules (red arrows), thus true negative on FES-PET/CT. FES-avidity in nodes (white arrow) is suspicious for malignancy. F, transaxial CT and fused FES-PET/CT demonstrates gastric avidity (blue arrow) suspicious for malignancy. G, transaxial CT and fused FES PET/CT demonstrates FES-avid osseous foci (yellow arrow) suspicious for malignancy. This osseous focus was subsequently biopsied and proved to be an osseous metastasis and thus true positive on FES-PET/CT.Image courtesy of JAMA Open Network
“No significant difference was found between FES PET/CT and current [standard-of-care] imaging for the detection of distant metastases in patients with ER-positive LABC or recurrences in patients with ER-positive BC and suspected recurrence,” the group wrote.
Ultimately, the study provides evidence that FES PET/CT performs comparably with current standard-of-care imaging methods for both of these clinical indications, the researchers concluded.
In an accompanying editorial, David A. Mankoff, MD, PhD, of the University of Pennsylvania, and colleagues wrote that the study data regarding staging patients with ILC is important to note. ILC is a subtype of breast cancer that is typically ER-positive and notoriously difficult to detect on imaging because it tends to grow in an infiltrative, less cohesive way, they wrote.
Further studies are warranted, including multisite trials, to support more routine use in clinical practice, they added.
“Overall, this study by Ulaner et al contributes important data to guide the use of new molecular imaging tools for [breast cancer] diagnosis and staging, contributing to the overall goal of precision breast oncology,” the editorial concluded.
The full study is available here.