PET/CT imaging with F-18 fluroestradiol (FES) can play a significant role helping clinicians make treatment decisions in patients with recurrent or metastatic breast cancer, according to a study published October 3 in the Journal of Nuclear Medicine.
In a large group of patients who underwent imaging between 2021 and 2023, F-18 FES-PET/CT supported clinical decisions in three quarters of cases and resulted in a management change in one third, noted lead author Jeongryul Ryu, MD, of the Asan Medical Center in Seoul, Korea, and colleagues.
“F-18 FES-PET/CT resulted in modification of the actual management of recurrent or metastatic breast cancer and can serve as an impactful imaging modality for determining the optimal treatment strategy,” the group wrote.
F-18 FES (Cerianna, GE HealthCare) is a PET radiotracer that binds to estrogen receptors in cancer cells and is highly effective for identifying cancer recurrence or metastases, according to the authors. The tracer was approved in 2020 and recently, appropriate-use criteria for the imaging agent were published by the Society of Nuclear Medicine and Molecular Imaging to promote its use, yet evidence on its clinical impact is still lacking, they noted.
To add to the available body of evidence, the group analyzed data from 344 patients with suspected or known recurrent or metastatic ER-positive cancer who underwent F-18 FES-PET/CT no more than three months after standard workups. Two experienced medical oncologists compared the planned clinical management for patients before and actual management after F-18 FES PET/CT via a medical chart review.
Ninety-nine patients (29%) were receiving adjuvant endocrine therapy after curative surgery, 51 (15%) were receiving palliative endocrine therapy, 16 (5%) were receiving neoadjuvant chemotherapy, 10 (3%) were receiving palliative chemotherapy, one (0.3%) was receiving adjuvant chemotherapy, one (0.3%) was receiving adjuvant radiotherapy, 81 (23%) were under regular follow-up after completion of treatment, and 85 (25%) had newly diagnosed breast cancer.
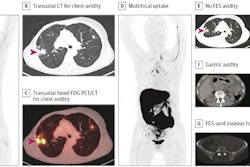
F-18 FES PET/CT supported existing management decisions without a change in management in 139 (40%) patients. One hundred and twenty (35%) patients experienced a change in management after F-18 FES PET/CT, for instance from palliative to curative treatment. Intention-to-treat and interdisciplinary changes accounted for 64% (77/120) and 68% (82/120) of all changes.
The highest rate of change was shown when the origins of metastasis of double primary cancers (64%, 9/14) were being evaluated.
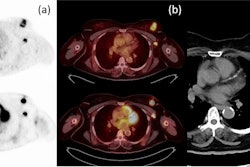
 A graphical abstract of the study. Image courtesy of the Journal of Nuclear Medicine.
A graphical abstract of the study. Image courtesy of the Journal of Nuclear Medicine.
“Our study included the largest (to our knowledge) prospective cohort of F-18 FES-PET/CT to date used in real-world practice after approval for clinical use,” the researchers wrote.
The five-year survival rate for patients with de novo metastatic breast cancer is only 31% and ER status is typically determined through biopsies and immunohistochemistry, yet biopsy is an invasive procedure, may result in sampling errors, and may not be feasible for all lesions, the group noted.
Further research is needed to determine whether treatment changes made after F-18 FES-PET/CT imaging results in better outcomes for patients, however.
“Finally, whereas F-18 FES-PET/CT was shown to lead to changes in management in a high percentage of patients, it is unknown whether these changes translated into better survival or quality-of-life outcomes,” the group concluded.
The full study is available here.