F-18 fluoroestradiol (FES) PET may improve staging of grade 1 or 2 estrogen receptor (ER)-positive breast cancer compared with F-18 FDG-PET, according to an article published March 4 in Radiology.
The finding is from a prospective pilot study and warrants future research to test whether FES PET/CT can be used as a tool for primary staging, noted lead authors Jelijn Knip, MD, PhD, and Ramsha Iqbal, MD, PhD, both of the Free University of Amsterdam in the Netherlands, and colleagues.
“We aimed to assess whether F-18 FES-PET could improve staging in low-grade ER-positive breast cancer. Despite the small sample size and the study not being powered to reach statistical significance, the results of this pilot study support this hypothesis,” the group wrote.
F-18 FES is a PET radiotracer approved by the U.S. Food and Drug Administration (FDA) in 2020 for detecting ER-positive lesions as an adjunct to biopsy in patients with advanced recurrent or metastatic breast cancer. Preliminary studies suggest the tracer may also be effective in patients with earlier, more treatable stages of the disease, yet most of these studies have been retrospective and subject to a large risk of bias, the authors noted.
Thus, the researchers aimed to determine prospectively whether 18F-FES PET improves staging for patients with clinical stage II/III or local-regional recurrent, grade 1 or 2, ER-positive breast cancer compared with standard F-18 FDG-PET.
Between December 2018 and January 2021, the group enrolled 41 female participants (median age, 56 years) who underwent both PET scans. Nuclear medicine physicians analyzed both sets of images and determined disease stages, with final stages verified via biopsy.
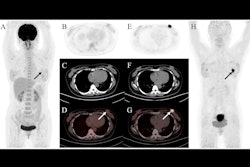
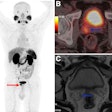
 (B) F-18 FDG-PET maximum intensity projection, F-18 FES-PET maximum intensity projection, breast MRI scan, and mammogram in an 81-year-old female participant who presented with a tumor in the right breast. F-18 FES-PET scan shows a second primary tumor (grade 1, lobular) in the left breast and malignant lymph nodes (T2N1M0) that are not visible on the F-18 FDG-PET scan. The tumor in the right breast (T4N0M0, grade 3 ductal carcinoma) is visible on both PET scans. All lesions had also been identified at mammography and MRI. Image and caption courtesy of the RSNA.
(B) F-18 FDG-PET maximum intensity projection, F-18 FES-PET maximum intensity projection, breast MRI scan, and mammogram in an 81-year-old female participant who presented with a tumor in the right breast. F-18 FES-PET scan shows a second primary tumor (grade 1, lobular) in the left breast and malignant lymph nodes (T2N1M0) that are not visible on the F-18 FDG-PET scan. The tumor in the right breast (T4N0M0, grade 3 ductal carcinoma) is visible on both PET scans. All lesions had also been identified at mammography and MRI. Image and caption courtesy of the RSNA.
Key findings included the following:
- Thirty-four of 41 (83%) participants were correctly staged with F-18 FES-PET compared with 29 of 41 participants (71%) with F-18 FDG PET (p = 0.18).
- Nine of 10 cases of lobular breast cancer were correctly staged with F-18 FES-PET compared with seven of 10 with F-18 FDG (p = 0.38).
- Eleven of 12 cases of grade 1 tumors were correctly staged with F-18 FES-PET compared with seven of 12 with F-18 FDG-PET (p = 0.06).
- Regional lymph nodes were incorrectly staged by F-18 FDG-PET in six of 44 cases (14%), whereas all lymph nodes were correctly staged with F-18 FES-PET (p = 0.02).
The next steps will be to confirm the study findings in a larger effort to determine the optimal role for F-18 FES PET, according to the authors. Ultimately, the goal is to assess whether the initial staging of ER-positive breast cancer should be added to the FDA’s clinical indications for the tracer, they noted.
“Using F-18 FES-PET as the primary staging modality instead of F-18 FDG-PET, especially in lobular and grade 1 breast cancer, could potentially lead to more correct staging and less unnecessary invasive diagnostics,” the group wrote.
In an accompanying editorial, Amy Fowler, MD, PhD, of the University of Wisconsin in Madison, echoed the call for additional studies and noted that a Society of Nuclear Medicine and Molecular Imaging (SNMMI) workgroup on appropriate use criteria recently concluded that additional research is needed before earlier use of the tracer can be recommended.
“If validated, the results of the study by Knip and Iqbal et al could help define the optimal target population in which to use FES PET for initial staging and could expand the currently approved clinical indications for updated practice guidelines and patient management,” she concluded.
The full study is available here.