A 21% increase in patient cases helped medical isotope developer IsoRay Medical to a third-quarter revenue gain and a lower net loss compared to the same quarter of the prior year.
For the quarter (end-March 31), IsoRay reported revenue of $1.4 million, a 17% increase compared to $1.2 million in the corresponding quarter of 2010. The company's net loss decreased from $1.1 million in the third quarter of 2010 to $1 million in 2011.
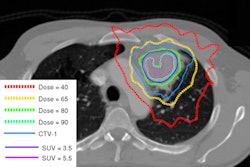
IsoRay's nonprostate cases represented 10% of overall sales, a factor attributed to increasing adoption of brachytherapy treatments for lung, brain, colon, head and neck, ocular melanoma, and prostate cancers. IsoRay is the exclusive manufacturer of cesium-131 used in these treatments.
In other developments, IsoRay received CE Mark certification for its stranded and mesh seed configurations of cesium-131 for cancer applications throughout the body. The company said it will now be seeking distributors of the product throughout Europe.
IsoRay also announced the launch of an e-clinical data system, an online Web data collection registry to examine the efficacy of brachytherapy compared to other techniques and treatments.