With 2.5 years under its belt, SNM's Clinical Trials Network is making progress on a number of fronts -- including two major clinical trials coming soon and increasing membership around the world -- that it hopes will contribute to the future of nuclear medicine and molecular imaging.
The Clinical Trials Network is a collaborative effort designed to address the need for validated imaging biomarkers that can be used in the development and registration of investigational therapeutics, particularly regarding the efficacy of molecular imaging biomarkers in clinical trials.
 Dr. Michael Graham, PhD, former SNM president and director of nuclear medicine at the University of Iowa.
Dr. Michael Graham, PhD, former SNM president and director of nuclear medicine at the University of Iowa.
Graham mentioned that the Clinical Trials Network is working with a major pharmaceutical company to begin two major clinical trials soon, but he declined to provide specific details for proprietary reasons.
"We are also talking to a number of the radiopharmaceutical manufacturers because they are interested in clinical trials support for their [molecular imaging] agents," he added. "The initial thrust was to utilize PET imaging to help assess response to therapy early in phase II trials for the large pharmaceutical company, with particular emphasis on [the PET tracer] fluorothymidine."
The Clinical Trials Network recently completed support of a small trial at 12 sites, and discussions are continuing with several other companies for future research.
Volunteer staff
While the Clinical Trials Network is likely to get a number of efforts up and running over the next year, Graham said the effort may be limited because of the volunteer nature of the organization.
"We need somebody to analyze the phantom images, and we need somebody to organize and at least make preliminary judgments on the sites," he added. "One of the things we want to do is look at image quality for the first few patients from the sites to assure that it is adequate. This is going to take a fair number of man-hours to do."
To address this, the Clinical Trials Network recently hired a PET/CT fellow at USC and an MD-PhD intern at Johns Hopkins University.
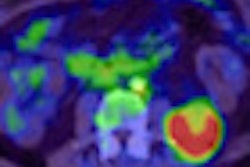
Most recently, the Clinical Trials Network's scanner validation committee certified its 100th PET/CT scanner as part of a program to ensure that sites involved in clinical trials have reliable, accurate, and reproducible PET image data. The critical issues are the quantitation of an image, the accuracy of measuring tumor standardized uptake values (SUVs), image quality, and lesion detectability.
The Clinical Trials Network uses one phantom that handles the technical measurements and provides a clinical simulator of disease. "We have a precise phantom with known quantitative accuracy, and the phantom also has lesions in locations unknown to the imaging site, which they have to identify and be able to measure quantitatively," said Paul Christian, committee chairman.
When pharmaceutical companies are testing therapeutic drugs for cancer, one of the primary elements in the progression of disease is to determine if any new lesions are present.
"It's not just measuring the quantitative SUV uptake before and after the drug therapy, but also [whether] new lesions are present," Christian added. "That's the advantage that our validation program has."
Custom-made phantom
The phantom was designed and developed by SNM's quality assurance committee, aided by Christian, who also serves as associate director for molecular imaging at the University of Utah's Huntsman Cancer Institute. The phantom is manufactured by Medical Designs of Newtown, CT, and is designed to help sites assess both equipment and personnel performance.
The custom-designed phantom simulates the chest of an oncology patient and provides quantitative accuracy of the concentration of radioactivity both within the body and within the disease. "When a site images the phantom, they should be able to see the lesion in a certain location, identify the location, and then be able to provide an accurate, quantitative measurement," Christian said.
 Paul Christian, chairman of the Clinical Trials Network's scanner validation committee.
Paul Christian, chairman of the Clinical Trials Network's scanner validation committee.
Because the Clinical Trials Network relies on a volunteer staff to carry out its duties and cannot send someone to each individual site for validation, it provides documentation on how to perform the studies, including how to load the phantom, image the phantom, and collect imaging data. There is also an online video that provides instructions.
"Some of these sites have never done clinical research studies in the past, and the documentation that is required by the drug companies and the [U.S. Food and Drug Administration (FDA)] is very substantial," Christian said. "It has to be done in a very proper way and recording must be accurate."
The Clinical Trials Network also has phantoms available for PET myocardial imaging and PET brain imaging. As of now, there are no research protocols for multicenter trials, but the phantoms have been developed for these applications "should a company come forward and want to partner with the Clinical Trials Network to provide these new imaging opportunities in PET," he added.
PET validation
As one might expect, the committee has found that PET scanners with newer technology perform better than older machines, which may require participating sites to change their imaging protocols.
Regardless of the age of the PET scanners in use, the systems "have to be properly maintained and properly calibrated," Christian said. "The image quality is driven by the user of the machine. We ensure that their imaging protocols are appropriate to provide a certain high level of image quality."
Ohio State University (OSU) Medical Center has had its two PET/CT scanners validated by the Clinical Trials Network and is looking to participate in new research projects.
"At this point, we are looking at it as a great opportunity to participate with SNM to help facilitate projects in the future," said Deborah Hurley, senior nuclear medicine technologist at OSU. "For us, it would be projects that may not be funded if it were not for the Clinical Trials Network."
NCCN connection
OSU is also part of National Comprehensive Cancer Network (NCCN), which recently announced a collaboration with the Clinical Trials Network to advance research for cancer imaging and therapies. OSU's Comprehensive Cancer Center draws patients from several states with a variety of different cancers and diagnoses.
"Our patient population lends itself to a good future as far as participating in those trials that will come through the network," Hurley said. "I think that it is advantageous for us to be part of the Clinical Trials Network because we will have a population of patients that will fit into some of these clinical trials that may not be seen at smaller hospitals."
Education is also among the Clinical Trials Network's priorities. Deborah Gibbs, supervisor for PET/CT and nuclear medicine at the Medical College of Georgia, has given several lectures through SNM to help bridge the communications gap between nuclear medicine technologists and clinical researchers.
Research nurses, for example, may not always understand the difference between a chest x-ray and a chest, abdomen, and pelvis scan, she said, and they may get bogged down in the terminology. To remedy that, the Medical College of Georgia scheduled in-service meetings between the nuclear medicine department and its researchers to "take down the walls," she said.
Quality control
Gibbs also has given lectures to technologists to help them participate in future clinical trials regarding standardized quality control. "In my lecture, I distinguish the differences between clinical trial quality control and daily quality control, why the specific techniques must be detailed and precise, and why it is critical that clinical trials quality control is done at specific intervals," she said. "If a technologist works [quality control] into the daily schedule, it will become second nature."
Gibbs also crafted spreadsheets and other resources to help technologists chart when a clinical trial patient is scheduled for a PET scan, when the research nurse requested the documentation or images, and other related information. "It is this kind of accountability that shows you have integrity in your department and nobody passes the buck," she added.
The greater good
The Clinical Trials Network is accepting all interested participants, regardless of the size of the imaging facility. PET Scan Arizona is a small, independent, single-modality imaging center that only performs PET/CT. The facility is involved in a small trial through the Clinical Trials Network, with a number of imaging centers across the country researching an imaging agent for breast cancer.
Terry Brown, executive director, said his facility's desire to be part of these burgeoning initiatives is driven by the center's objectives, goals, and mission. "It enhances the employment experience of our technologists in the center. It is a way to give back to the medical community," he added. "I feel it is something we should do, when given the opportunity."