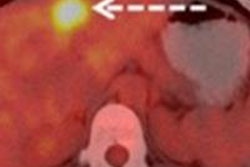
CHICAGO - FDG-PET/CT's ability to evaluate pathological response after two cycles of neoadjuvant chemotherapy could help breast cancer patients avoid ineffective treatment, according to a study presented on Sunday at the RSNA 2011 meeting.
Neoadjuvant chemotherapy can shrink a tumor mass in breast cancer patients, potentially reducing the need for surgery, noted lead author Dr. Carolin Riegger, from the University of Dusseldorf 's department of diagnostic and interventional radiology, who presented the study results. However, approximately 25% of patients do not respond to such therapy. Early differentiation between people who respond to treatment and those who don't can help avoid ineffective treatment and reduce unnecessary side effects.
The study included 26 female breast cancer patients (mean age, 46.9 years ± 9.9), who received whole-body FDG-PET/CT at baseline and again after their second cycle of neoadjuvant chemotherapy.
Riegger and colleagues rated patient tumor response after two rounds of chemotherapy on a scale of 0 to 4, with 0 meaning treatment had no effect and 4 signifying a satisfactory response and the absence of the tumor. Histopathology of the resected tumor following neoadjuvant chemotherapy served as the reference standard to differentiate between complete pathological response (4) and partial or no pathological response (0-3).
The researchers also measured maximum standardized uptake values (SUVmax) of breast cancer lesions before therapy, as well as SUVmax after the second cycle of neoadjuvant chemotherapy. The group then compared the absolute and relative decline of SUVmax between the two measurements for both groups of patients (responders and nonresponders).
According to histopathology, four patients had a complete pathological response to chemotherapy, while 22 patients had no response. The SUVmax before neoadjuvant chemotherapy was 15.1 in the pathologically responsive group and 7.9 in the nonresponsive group. The results were not statistically significant (p = 0.055), according to the researchers.
Two patients had a score of 0, with an SUVmax of 2.8 before treatment and an SUVmax of 2.6 after two rounds of chemotherapy. Ten patients had a score of 1, with an SUVmax of 14.6 prior to therapy and an SUVmax of 12.4 after treatment.
Six patients had a score of 2, with an SUVmax of 2.9 before chemotherapy and an SUVmax of 1.5 after treatment. Four patients had a score of 3, with an SUVmax of 19.7 prior to treatment and an SUVmax of 6.6 after chemotherapy. Finally, four patients had a score of 4, with an SUVmax of 23.9 before therapy and an SUVmax of 2.1 after the two-round regimen.
The SUVmax after the second cycle of neoadjuvant chemotherapy was 1.7 in the group with a pathological response and 3.0 in the group with no response. This difference also was not statistically significant (p = 0.126).
The absolute and relative declines of SUVmax in the complete pathological response group were 13.4 and 84%, respectively, compared with 4.9 and 50% for the group with no pathological response. These differences were statistically significant (p < 0.05).
FDG-PET/CT is able to differentiate between complete pathological response and no complete pathological response in primary breast cancer lesions after two cycles of neoadjuvant chemotherapy, Riegger and colleagues concluded.
The modality's ability to make this differentiation in the early stages of neoadjuvant chemotherapy "may offer the opportunity to adapt therapy planning at an early stage in nonresponding patients and to avoid ineffective treatment," the authors wrote.