While guidelines recommend FDG-PET/CT only as an optional workup for women with stage III breast cancer, the hybrid modality can help women younger than 40 who have potentially aggressive stage II breast cancer, according to a study published online September 11 in the Journal of Nuclear Medicine.
By extending the use of FDG-PET/CT to this patient population, researchers at Memorial Sloan Kettering Cancer Center discovered stage IV disease in 10% of patients younger than 40 years who had an initial diagnosis of stage I or stage II breast cancer. In addition, there were distant metastases in 17% of asymptomatic stage IIB breast cancer patients.
"This study looked at young women who have more-aggressive breast cancer," said co-author Dr. Gary Ulaner, assistant attending radiologist in Memorial Sloan Kettering's department of radiology, molecular imaging, and therapy services. "Therefore, it seemed possible that FDG-PET/CT may be of value earlier than stage III breast cancer. The data that we present suggests that indeed it is."
NCCN guidelines
The National Comprehensive Cancer Network (NCCN) recommends that FDG-PET/CT only be used for stage III breast cancer patients. However, there has been some debate, even among radiologists and clinicians at Memorial Sloan Kettering, as to whether TNM (tumor, node, and metastasis) staging should be the only criteria.
 Dr. Gary Ulaner from Memorial Sloan Kettering Cancer Center.
Dr. Gary Ulaner from Memorial Sloan Kettering Cancer Center.Based on previous studies, Ulaner estimated that FDG-PET/CT detects distant metastases in approximately 25% of all patients with locally advanced breast cancer. The results will often change their status from locally advanced stage III to metastatic stage IV breast cancer and, in turn, alter treatment and prognosis.
In addition, women diagnosed with breast cancer at a younger age tend to have more aggressive forms of the disease.
"Our study says that for these younger women, FDG-PET/CT not only should be used for systemic staging for stage III, but also for some stage II patients who may benefit from FDG-PET/CT because their tumors are probably more aggressive and metastasize earlier," Ulaner said.
Lead author Dr. Christopher Riedl, Ulaner, and colleagues reviewed the facility's records for stage I through stage IIIC breast cancer patients younger than 40 years old who received FDG-PET/CT scans prior to treatment at the institution between January 2003 and December 2012 (JNM, September 11, 2014).
The researchers excluded patients with known stage IV breast cancer before FDG-PET/CT, prior malignancies except for nonmelanoma skin cancer, and treatment before FDG-PET/CT such as local radiation therapy or curative surgery.
The median age of the 134 patients was 36.2 years, ranging from 22.2 to 39.9 years. The median time between initial diagnosis and FDG-PET/CT was 21.5 days, ranging from 0 to 112 days.
Initial breast cancer staging was based on a physical exam, as well as mammography, ultrasound, and breast MRI results. FDG-PET/CT was then used to identify any unsuspected regional nodal and distant metastases.
Before FDG-PET/CT, 15% of the 134 patients were classified as having stage I breast cancer, 33% were stage IIA, 35% were stage IIB, and 17% were stage III. The majority of patients had invasive ductal cancers (92%), and most of the cancers were grade 3 tumors (82%).
FDG-PET/CT discoveries
FDG-PET/CT identified unexpected extra-axillary regional nodal and distant metastases in 28 (21%) of the 134 patients. Fifteen patients (11%) had extra-axillary regional nodal metastases and 20 (15%) had distant metastases; seven patients had both regional nodal and distant metastases.
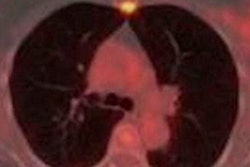
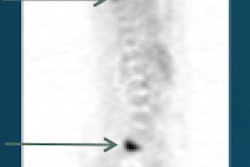
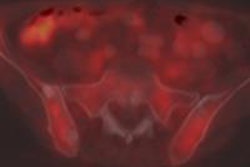
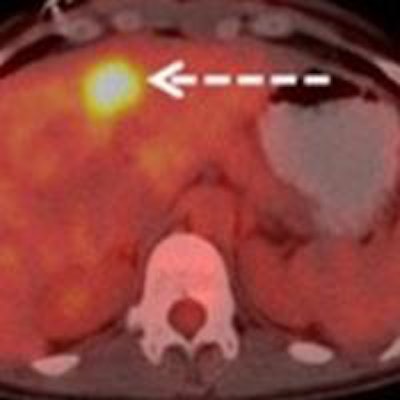
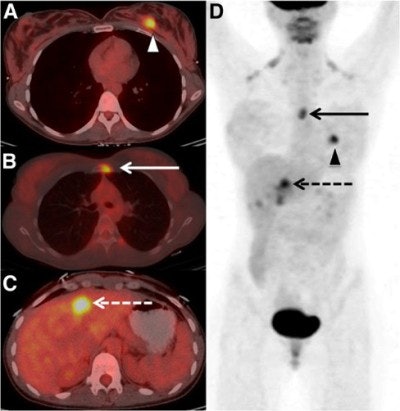 Images are from a 29-year-old woman with stage IIA breast cancer that was upstaged to stage IV by FDG-PET/CT. Axial PET/CT images showed known (A) primary left breast cancer (arrowhead), previously unknown (B) osseous metastasis (solid arrow), and previously unknown (C) liver metastasis (dashed arrow) proved by biopsy. PET also provided an overview of all lesions (D). Images courtesy of JNM.
Images are from a 29-year-old woman with stage IIA breast cancer that was upstaged to stage IV by FDG-PET/CT. Axial PET/CT images showed known (A) primary left breast cancer (arrowhead), previously unknown (B) osseous metastasis (solid arrow), and previously unknown (C) liver metastasis (dashed arrow) proved by biopsy. PET also provided an overview of all lesions (D). Images courtesy of JNM.The 20 women with distant metastases were upgraded to stage IV. Sixteen patients had osseous metastases, six had distant nodal metastases, five had liver metastases, two had lung metastases, and one had a splenic metastasis. Seven patients had more than one distant metastatic site.
FDG-PET/CT upgraded to stage IV one woman out of 20 who had an initial diagnosis of stage I. In addition, two of 44 stage IIA patients, eight of 47 stage IIB patients, four of 13 stage IIIA patients, four of eight IIIB patients, and one of two stage IIIC patients were changed to stage IV.
There were no false positives among the lesions suspicious for distant metastasis, as all lesions were confirmed by biopsy.
"Our data suggest that FDG-PET/CT identifies clinically unsuspected stage IV disease in 10% of women younger than 40 years with initial clinical stage I and II breast cancers, a population not currently recommended to undergo FDG-PET/CT," the authors wrote.
Unexpected findings
Ulaner said he and his colleagues were somewhat surprised that FDG-PET/CT discovered previously unknown malignancies in five of the women in the study. Four synchronous thyroid malignancies and one rectal malignancy were detected.
"So in addition to benefiting them for staging their known breast cancer, we also found another malignancy that they needed to be evaluated for," he said.
Despite the results, there is still some debate at Memorial Sloan Kettering over when to use FDG-PET/CT for this younger population of breast cancer patients.
"Some physicians have adopted a policy whereby young women less than 40 years old with stage IIB [breast cancer] who previously would not have warranted an FDG-PET/CT scan now do receive it, but I can't say that [policy] is universal," Ulaner said.
The researchers are now examining patient factors other than age to try to define when FDG-PET/CT is most appropriate for staging newly diagnosed breast cancer.