FDG-PET/CT's ability to find a significant number of distant metastases in patients with newly diagnosed stage IIB triple-negative breast cancer suggests that current guidelines from the National Comprehensive Cancer Network (NCCN) for the modality's use may need to be revised.
Researchers from Memorial Sloan Kettering Cancer Center (MSKCC) used FDG-PET/CT to find unsuspected distant metastases in 15% of patients with initial stage IIB triple-negative breast cancer. The NCCN recommends that FDG-PET/CT be used to stage patients with newly diagnosed stage III triple-negative breast cancer.
"Our findings suggest that FDG-PET/CT may be useful in staging even earlier triple-negative breast cancer," said lead author Raychel Castillo in a presentation earlier this month at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting. "This demonstrates that FDG-PET/CT as a diagnostic test was able to find more advanced disease than was previously expected."
NCCN guidelines
While the NCCN suggests that FDG-PET/CT be considered for the systemic staging of initial clinical stage III breast cancer patients, other factors such as receptor phenotype may influence when the hybrid modality is used in assessing breast cancer, Castillo said.
"Triple-negative breast cancer lacks all three of the known receptors: estrogen, progesterone, and HER2 receptors," she added. "This type of breast cancer tends to be more aggressive and metastasizes earlier than other breast cancers."
Thus Castillo and colleagues hypothesized that receptor status of a breast malignancy may be one key characteristic in determining which groups of patients might benefit from FDG-PET/CT staging at initial diagnosis.
The MSKCC researchers retrospectively reviewed the facility's records to screen for patients who underwent FDG-PET/CT scans for triple-negative breast cancer between 2007 and 2013 prior to systemic or radiation therapy.
There were 232 subjects who met the study inclusion and exclusion criteria. Their stages were assessed through ultrasound, mammography, MRI, and biopsy results prior to FDG-PET/CT imaging.
The vast majority of patients were diagnosed with either stage IIA (82 patients, 35%) or stage IIB (87 cases, 38%) breast cancer, followed by stage I (23 patients, 10%) and stage IIIA (23 patients, 10%). There were 217 women (94%) with invasive ductal carcinoma, with the same number of subjects (217, 94%) having a high tumor grade.
FDG-PET/CT images were then reviewed again to look for any unsuspected, extra-axillary regional nodal metastases and distant metastases, as well as synchronous malignancies, Castillo said. Distant metastases were confirmed through histology.
Upstaging cancer
The researchers found unsuspected distant metastases in 30 patients (13%), which prompted the upstaging of their condition to stage IV triple-negative breast cancer. In addition, five unsuspected synchronous malignancies were discovered.
Seventeen women (10%) with an initial diagnosis of stage II triple-negative breast cancer were upgraded to stage IV, including 13 patients (15%) initially at stage IIB.
"This finding suggests that FDG-PET/CT may also be useful in evaluating patients with initial clinical stage IIB triple-negative breast cancer," Castillo said.
| FDG-PET/CT staging results | ||
| Initial clinical stage | No. of subjects | Upstaged to stage IV |
| I | 23 | 0 (0%) |
| IIA | 82 | 4 (5%) |
| IIB | 87 | 13 (15%) |
| IIIA | 23 | 4 (17%) |
| IIIB | 14 | 8 (57%) |
| IIIC | 3 | 1 (33%) |
| Total | 232 | 30 (13%) |
Among women with initial diagnoses of stage III triple-negative breast cancer, 13 (32%) were upgraded to stage IV.
"As suspected, patients who had initial stage III disease had a high rate of upstaging after PET/CT images were reviewed," Castillo said. "This finding supports the NCCN recommendations to consider the use of FDG-PET/CT in the evaluation of patients with initial clinical stage III breast cancers."
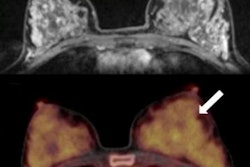
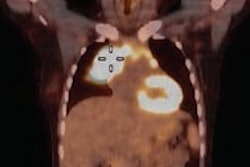
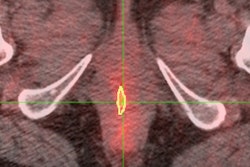
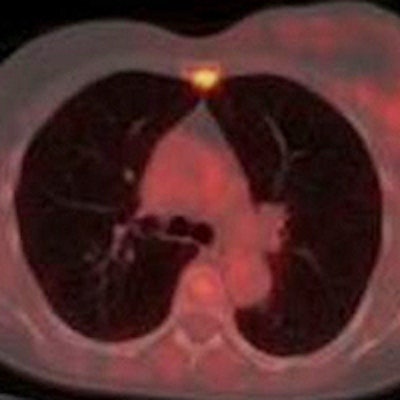
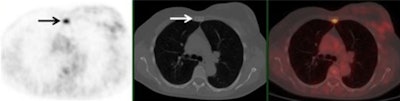 A 61-year-old woman with initial stage IIB triple-negative breast cancer was upgraded to stage IV. Axial PET, CT, and fused FDG-PET/CT show an FDG-avid osseous metastasis without CT correlate (arrows). A biopsy confirmed the metastasis. Image courtesy of Raychel Castillo and the SNMMI.
A 61-year-old woman with initial stage IIB triple-negative breast cancer was upgraded to stage IV. Axial PET, CT, and fused FDG-PET/CT show an FDG-avid osseous metastasis without CT correlate (arrows). A biopsy confirmed the metastasis. Image courtesy of Raychel Castillo and the SNMMI.Given the results, Castillo and colleagues concluded that FDG-PET/CT can be used as a diagnostic tool to find more advanced disease than in triple-negative breast cancer patients with stage II disease, thus slightly altering current NCCN guidelines.
At the SNMMI meeting, the paper received an award for Best SNMMI Abstract Involving Breast Cancer Research.