Radiopharmaceutical developer Cell>Point has signed a long-term collaboration agreement including product licenses for Brazil with MJM Produtos Farmacêuticos e de Radioproteção (Radiopharmacus).
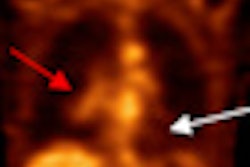
The agreement covers Cell>Point's technetium-99m-labeled glucosamine (Tc-99m EC-G) cancer and cardiology molecular imaging agent, along with other products being developed, the firm said.
In addition to the exclusive right to manufacture and sell Cell>Point products in Brazil following regulatory approval, Radiopharmacus will also provide services including commercial scale synthesis, certified good manufacturing practice (cGMP) manufacturing, preclinical and clinical studies, and regulatory activities.