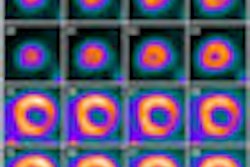
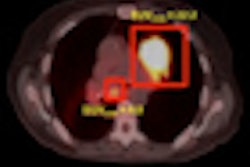
Bracco Diagnostics' recall of its CardioGen-82 cardiac PET radiopharmaceutical is officially over.
In a podcast on February 1, the U.S. Food and Drug Administration (FDA) confirmed that improper use of CardioGen-82 at two clinical sites caused three patients to be exposed to more radiation than typically associated with a CardioGen-82 scan.
Lesley Navin, a consumer safety officer in the FDA's Division of Drug Information, said the increased radiation exposure was due to administration of CardioGen-82 generator eluates that contained excess concentrations of strontium-82 (Sr-82) and strontium-85 (Sr-85), a phenomenon known as "strontium breakthrough."
The FDA believes it is "unlikely that this excessive exposure posed significant risks to patients, though exposure to any excessive radiation is undesirable," she added.
In July 2011, the FDA began investigating whether Bracco's manufacturing procedures for CardioGen-82 may have caused the incidents. However, the agency concluded this week that the company was not to blame for the excess radiation.
"The recalled CardioGen-82 generators that were functional following shipping were tested by the manufacturer to identify potential structural or functional causes of strontium breakthrough," Navin said. "None of the tested generators showed signs of breakthrough."
For several months now, Bracco has been in contact with its customers across the U.S. to assess the extent to which patients may have been exposed to excessive radiation.
According to the FDA, preliminary data show that of 375 patients who were surveyed at 43 clinical sites, 54 patients were planned for further radiation testing because of abnormal test results. All 54 patients are from two clinical sites. "Both sites appear to have insufficient documentation of compliance with the CardioGen-82 labeling recommendations for strontium breakthrough testing," Navin added.
The FDA is also working with Bracco to enhance the labeling for CardioGen-82 to better describe how to use the generator and to implement a plan for returning CardioGen-82 to the market.