Using PET to image the radiopharmaceutical FDDNP in the brain, researchers at the University of California, Los Angeles (UCLA) are showing how the accumulation of abnormal protein deposits is associated with the progression of Alzheimer's disease, according to a study in the February issue of Archives of Neurology.
Among 21 subjects with mild cognitive impairment, FDDNP-PET showed greater levels of plaque and tangled protein deposits in the frontal and parietal areas of the brain, which are involved with decision-making, reasoning, memory, and emotions. By viewing these areas, researchers were able to track and predict the cognitive decline of those individuals over a two-year period (Arch Neurol, Vol. 69:2, pp. 215-222).
The UCLA research team, led by Dr. Gary Small, UCLA's Parlow-Solomon Professor on Aging and a professor of psychiatry at the Semel Institute for Neuroscience and Human Behavior, developed the chemical marker FDDNP that binds to both plaque and tangle deposits.
Small noted that FDDNP-PET is the only available brain imaging technique that can assess the protein deposits, also known as tau tangles. Autopsy results have found that the tau tangles correlate with Alzheimer's disease progression much better than levels of plaque.
The study enrolled 43 volunteers with an average age of 64 (range, 40 to 87 years) who had no signs of dementia. Thirty participants, however, had a family history of dementia in at least one close relative. At the start of the study, 22 participants exhibited normal aging patterns, while the other 21 had mild cognitive impairment.
The researchers performed brain scans with FDDNP-PET (Exact HR+, Siemens Healthcare) and conducted cognitive assessments on the subjects at baseline and two years later.
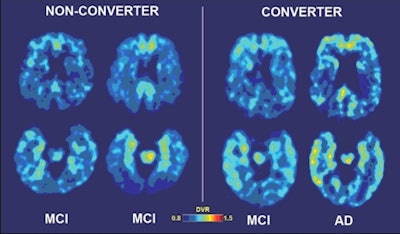 |
| Baseline and follow-up brain scans of a patient who developed Alzheimer's disease after two years (images to right) show high medial temporal binding at baseline (lower left) and follow-up (lower right), as well as more baseline binding in frontal (upper images) and lateral temporal regions. Yellow areas indicate higher binding levels. Scans of a second patient who did not develop Alzheimer's after two years (images to left) show medial temporal binding (lower scans) but very mild frontal (upper scans) binding at baseline and follow-up. Images courtesy of UCLA. |
At the two-year follow-up, the 21 subjects with mild cognitive impairment showed significant increases in FDDNP uptake in frontal, parietal, posterior cingulate, and global regions of the brain. The group of 22 normal individuals showed no significant increases in any region of the brain.
FDDNP binding in those key areas correlated with increases in clinical symptoms over time, and initial binding levels were predictive of future cognitive decline.
Among the 21 subjects with mild cognitive impairment, the level of initial binding in the frontal and parietal areas of the brain provided the greatest accuracy in identifying those who developed Alzheimer's disease after two years, Small and colleagues noted. Of the 21 subjects, six were diagnosed with Alzheimer's at the two-year follow-up; all six had higher initial frontal and parietal binding values than other subjects in the mild cognitive impairment group.
Among the normal aging subjects, three developed mild cognitive impairment after two years. Two of those participants had the highest baseline binding values in the temporal, parietal, and frontal brain regions within the group.
The researchers plan to extend their study to include a longer duration of follow-up with larger samples of subjects. In addition, Small and colleagues plan to use FDDNP-PET in clinical trials to help track novel therapeutics for brain aging, such as curcumin, a chemical found in turmeric spice.
Due to FDDNP's ability to predict oncoming dementia, the technique may be particularly useful in tracking the effectiveness of interventions designed to delay the onset of dementia symptoms and eventually prevent the disease, Small added.