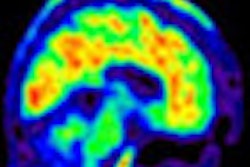
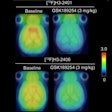
While the Alzheimer's Association supports the U.S. Food and Drug Administration's (FDA) recent clearance of Eli Lilly's Amyvid (florbetapir) radiopharmaceutical to view amyloid plaque in the brain, the organization is calling the use of the PET imaging agent a "double-edged sword."
In a prepared statement, the association acknowledged the expanded clinical and research opportunities that will result from using florbetapir imaging of amyloid plaques, which have been linked to Alzheimer's and dementia.
However, the "fact that all of the potential uses of this product are not crystal clear tempers our enthusiasm," the organization stated. "Again, additional research is needed to clarify the role of florbetapir-PET imaging in Alzheimer's."
To further investigate florbetapir's potential, the organization has created a task force with the Society of Nuclear Medicine to develop recommendations for the use of amyloid imaging for physicians, imaging, and other medical specialists, as well as Alzheimer's families and the general public.
The association also stated that it is concerned about the possibility of "less than scrupulous operators offering imaging services and making unrealistic promises about the value of florbetapir imaging to sometimes vulnerable and worried individuals."
As a result, the organization recommends that people have the test only as part of a complete evaluation of medical/neurological status and with appropriate expert consultation.
Despite the concerns, the Alzheimer's Association added that it believes Amyvid "is valuable to the Alzheimer's field -- to the pursuit of better Alzheimer diagnostics, treatments and preventions -- to have this product more widely available."