GE Healthcare has received U.S. Food and Drug Administration (FDA) clearance for its Q.Freeze PET/CT quantitative imaging software.
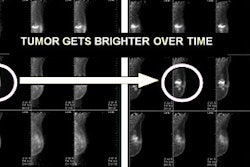
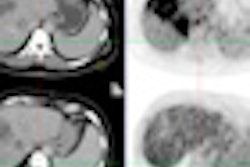
Q.Freeze is designed to improve quantitative consistency over conventional static PET imaging techniques and to facilitate reading of 4D PET/CT imaging. The software combines the quantitative benefits of GE's MotionMatch 4D phase-matched PET/CT imaging technology into a single static image, according to the vendor. CT and PET data are collected at each phase of the breathing cycle and then matched for attenuation correction purposes, GE said.
No acquisition data are wasted, as 100% of the counts collected are combined to create the static image, GE said. Q.Freeze is part of GE's Q.Suite portfolio of quantitative PET applications.