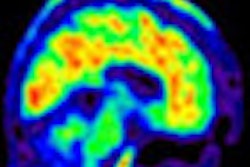
Imaging agent developer FluoroPharma Medical is touting results from an investigator-sponsored clinical trial in China in which patients with coronary artery disease were given the company's BFPET radiopharmaceutical to measure cardiovascular blood flow.
BFPET is an F-18-labeled tracer designed to enter myocardial cells in direct proportion to blood flow and cell membrane potential. BFPET has been designed to differentiate between cells of the myocardium that may be ischemic or infarcted and cells that are healthy.
Patients are being imaged at the PLA 301 hospital in Beijing, where images give a direct comparison between stress perfusion imaging using sestamibi and BFPET. The trial is expected to include approximately 20 patients and will conclude by the start of this year's fourth quarter.
Dr. Alan Fischman, former head of nuclear medicine at Massachusetts General Hospital and the principal investigator of the BFPET phase I trial, said that BFPET images so far have shown clear diagnostic qualities as well as enhanced resolution. The group is confident that the data will support further clinical development of the agent, he added.
FluoroPharma was granted patent rights in China for BFPET last year, as well as for another imaging agent, AZPET, which is in the very early phase of discovery.