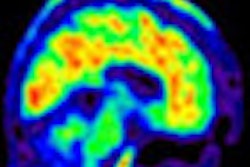
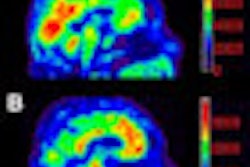
The U.S. Food and Drug Administration (FDA) is admonishing Eli Lilly for posting what it called "misleading" multicolored images on its website illustrating results with the PET radiopharmaceutical Amyvid (florbetapir F-18 injection).
Amyvid has received FDA clearance for PET imaging of the brain to estimate beta-amyloid plaque, which has been linked to cognitive impairment and Alzheimer's disease.
However, the letter from the FDA's Office of Prescription Drug Promotion (OPDP) stated that the "presentation misleadingly suggests that Amyvid PET images can be displayed, and therefore interpreted, in color in patients with cognitive impairment who are being evaluated for Alzheimer's disease and other causes of cognitive decline, when this is not the case."
The FDA stipulates that Amyvid PET scans should be displayed and reviewed "using a black-white scale with the maximum intensity of the scale set to the maximum intensity of all the brain pixels."
Lilly also displayed the same multicolored images in April at the company's exhibit at the American Academy of Neurology annual meeting in New Orleans.