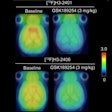
The U.S. Centers for Medicare and Medicaid Services (CMS) will convene an advisory committee meeting in January 2013 to review its policies regarding coverage of PET imaging studies of beta amyloid in the brain.
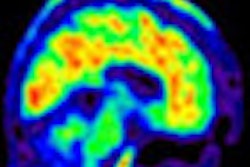
The agency's Medicare Evidence Development and Coverage Advisory Committee (MEDCAC) will meet on January 30 to review PET for beta-amyloid imaging, which is typically indicated for patients with neurodegenerative pathology such as Alzheimer's disease.
CMS is most interested in the exam's effects on patient function and quality of life. The agency will also seek MEDCAC's input on whether or not the published literature identifies patient characteristics that predict improved health outcomes of patients who undergo PET for beta-amyloid identification.
Medicare does not currently cover beta-amyloid PET imaging, but at least one new beta-amyloid radiotracer, Amyvid from Eli Lilly subsidiary Avid Radiopharmaceuticals, was approved by the U.S. Food and Drug Administration in 2012.
The meeting will be held at the CMS facility in Baltimore; the announcement can be viewed by clicking here.