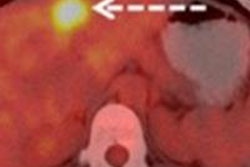
By using FDG-PET/CT, physicians will be in a better position to noninvasively predict a breast cancer patient's response to treatment after two cycles of neoadjuvant chemotherapy, helping them decide on the next course of action, concluded a study from Germany.
Lead author Dr. Till-Alexander Heusner, from the department of diagnostic and interventional radiology at the University of Düsseldorf, presented the study's results at this year's European Congress of Radiology (ECR) in Vienna.
Previous research has found that neoadjuvant chemotherapy reduces tumor mass in breast cancer lesions and enables surgeons to offer breast conservation surgery, Heusner noted in his talk. In addition, treatment with neoadjuvant chemotherapy diminishes the risk of intraoperative tumor cells.
"The histopathological response provides the longest survival information compared to other patients, but, unfortunately, we have 2% of patients who do not have an adequate response," he said. "An early differentiation between responders and nonresponders [to neoadjuvant chemotherapy] would be desirable."
In the study, Heusner and colleagues investigated whether FDG-PET/CT could differentiate between lesions with and without a pathological complete response after two cycles of neoadjuvant chemotherapy. They also wanted to determine the optimum maximum standardized uptake (SUVmax) value for the groups.
To correlate the FDG-PET/CT findings with a patient's histopathology, researchers used the Sinn score, which ranges from 0, meaning treatment had no effect on a tumor, to 4, meaning no viable tumor is detected.
They also created two algorithms to compare results between patients who responded to neoadjuvant chemotherapy and those who did not respond: One algorithm defined a pathological complete response as a Sinn score of 3 and 4, while the other algorithm defined a pathological complete response as a Sinn score of 4.
Heusner and colleagues also calculated the SUVmax of cancer lesions before treatment and after the second cycle of neoadjuvant chemotherapy.
The patient cohort consisted of 26 women (mean age, 46.9 years ± 9.9) with histologically proven breast cancer. Most of the patients had T2 stage cancer and grade II or III tumors; 21 (81%) of the 26 patients had ductal carcinomas.
All participants underwent a full-dose, contrast-enhanced FDG-PET/CT scan prior to neoadjuvant chemotherapy, and they received a low-dose FDG-PET/CT scan after the second cycle of neoadjuvant chemotherapy. Histopathology after the second round of treatment served as the gold standard for reference.
The authors determined the absolute and relative decline in SUVmax for patients with or without a pathological complete response.
With the algorithm using Sinn scores 3 and 4, the relative change in SUVmax was 47% in the nonpathological-complete-response group, compared with 79% for the pathological-complete-response group. The difference was statistically significant, according to Heusner and colleagues.
Using the algorithm with a Sinn score of 4 only, the relative change in SUVmax was 59% in the nonpathological-complete-response group, compared with 89% in the pathological-complete-response group. Once again, the difference was statistically significant.
Based on a receiver operator curve (ROC) analysis, an 88% change in SUVmax was the optimal cutoff value to differentiate between lesions with and without a pathological complete response, the group found.
"FDG-PET/CT may noninvasively predict the histopathological complete response after two cycles of neoadjuvant chemotherapy for breast cancer patients," the researchers concluded. "This may have a clinical impact on the decisions regarding neoadjuvant chemotherapy."
Heusner cited several limitations in the study, including the relatively low number of patients.