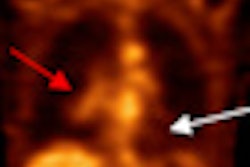
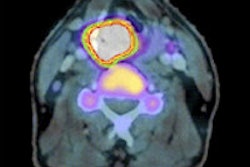
Radiopharmaceutical developer Cell>Point said that results from phase I and IIa studies of its technetium-99m-labeled ethylenedicysteine-glucosamine (99mTc-EC-G) nuclear imaging contrast agent have been favorable for evaluating the presence and severity of coronary artery disease.
Next up is a multicenter phase IIb trial, which is designed to evaluate the diagnostic accuracy of a 99mTc-EC-G study performed at rest only in comparison with a two-day 99mTc-Cardiolite rest/stress study to determine the presence, anatomical location, and severity of ischemia in patients with coronary artery disease, according to the company.
The phase IIb study is being led by investigator Dr. Gary Heller, a professor of medicine and nuclear medicine at the University of Connecticut School of Medicine.
In a statement, Cell>Point President Dr. David Rollo said that, based on the clinical data for phase I and IIa and assuming further confirmation in phase IIb, the company believes 99mTc-EC-G could lead to significant shortening of procedure times, increased diagnostic accuracy, and an improved patient experience by eliminating the need in most cases for a separate stress study.