Absolute Imaging Solutions (AIS) has received U.S. Food and Drug Administration (FDA) 510(k) clearance for a SPECT camera developed by Hungarian device firm Mediso Medical Imaging Systems.
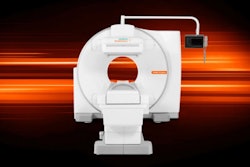
The clearance covers Mediso's AnyScan S general-purpose SPECT camera, available in single- and dual-head large field-of-view configurations. The camera line offers an open-design gantry, variable-angle detector positions, and a small footprint, among other features.
It also includes a table that supports patients up to 500 lb and preprogrammed robotic gantry motions with full automatic motion positioning and calibration.
AIS will introduce the AnyScan S line at the southeastern chapter of the Society of Nuclear Medicine and Molecular Imaging (SNMMI) 2013 meeting in Charlotte, NC.
The firm will begin shipping AnyScan S in the fourth quarter of 2013.