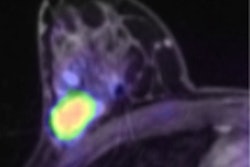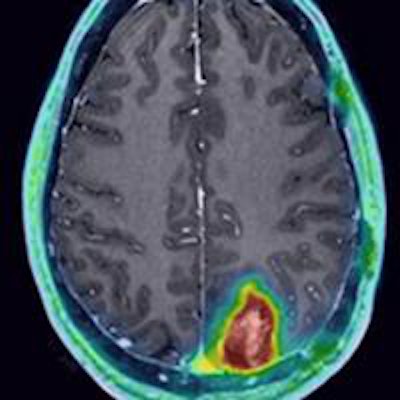
Researchers at the University of Wisconsin's UW Health system have completed the first successful human clinical trial of a new PET radiopharmaceutical that targets and detects brain tumors more specifically than FDG.
Iodine-124 (I-124) CLR1404 is designed to enter cells through membrane lipid rafts, which are overexpressed in cancer cells and serve as a platform for cell proliferation. CLR1404 is being developed as a diagnostic and therapeutic imaging agent for multiple solid tumors.
"This agent is taken up by cancer stem cells, which could be phenomenal in a therapeutic application," said Dr. Lance Hall, an assistant professor of radiology at UW Health. "Cancer stem cells are inherently resistant to radiotherapy and chemotherapy. If you leave behind as few as 100 cancer stem cells, patients often have [disease] progression and a more aggressive form of the disease."
Preclinical studies
For many years, UW Health researchers have been testing CLR1404 uptake and retention using more than 50 preclinical tumor models. The research has been led primarily by Jamey Weichert, PhD, an associate professor in UW Health's department of radiology. Weichert also started a private company called Cellectar Biosciences (formerly Novelos Therapeutics) to develop novel imaging agents such as CLR1404.
Hall, who is the physician sponsor for the investigational new drug (IND) application for CLR1404, joined the research efforts about five years ago.
"We have cured cancer 1,000 times in animals diagnosed with everything we could think of, but when you translate that to human studies, you start to see a lot of problems we did not anticipate," he said in a conversation with AuntMinnie.com.
Hall noted limitations in current imaging techniques for both metastatic and primary brain tumors. MRI, for example, does not always offer a complete delineation of tumor infiltration, which, in turn, could adversely affect surgical resection of tumors or radiotherapy planning.
While FDG is a mainstay in oncology imaging, it can be difficult at times to differentiate between malignant cells and inflammation.
"What we have seen in preclinical models and want to prove in humans is that [CLR1404] is more cancer-specific and targets cancers rather than inflammation or false positives that you may see with FDG-PET scans," Hall said. "We want to develop an agent that could be used to predict tumor response to therapy. If we have an imaging agent that says whether a person will respond to a therapeutic dose or not, we won't waste time and money."
Image comparisons
Hall presented results from CLR1404's first human clinical trial at last month's Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
The cohort included 16 patients with primary or metastatic brain tumors. Eleven of the patients were diagnosed with World Health Organization (WHO) grade II to grade IV gliomas. One patient was newly diagnosed with brain cancer and 15 were evaluated for tumor recurrence or whether a treatment change was warranted.
The subjects were injected with 2 to 5 mCi (74 to 185 MBq) of CLR1404 and imaged with PET/CT six hours, 24 hours, and 48 hours after injection. Fourteen patients were followed until surgery or with gadolinium-enhanced MRI for six months or longer.
The researchers found avid CLR1404 uptake in 12 of the 16 patients; four individuals with cancer recurrence (as opposed to pseudoprogression) had no tracer uptake. There was no significant uptake of CLR1404 in normal areas of the brain and no uptake in regions that were treated for cancer and were presumably tumor-free.
CLR1404-PET successfully imaged tumors with high tumor-to-background uptake and uncovered larger tumor volumes than contrast-enhanced MRI.
"We saw areas of concordant and discordant PET uptake and MRI enhancement," Hall said. "This is what we are really excited about, those discordant areas. Those are the areas where we have the ability to add perhaps more accurate complementary information to what MRI shows."
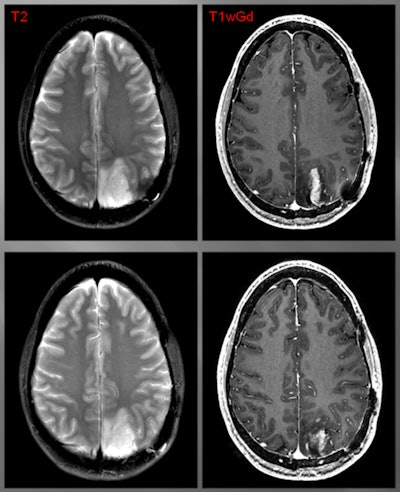 Patient with a grade III glioma is imaged with T2-weighted MRI (above left). Follow-up gadolinium-enhanced MRI (above right) suggested cancer recurrence. CLR1404-PET detected cancer (below left) and fused PET/MRI (below right) delineated the tumor. All images courtesy of Dr. Lance Hall and UW Health.
Patient with a grade III glioma is imaged with T2-weighted MRI (above left). Follow-up gadolinium-enhanced MRI (above right) suggested cancer recurrence. CLR1404-PET detected cancer (below left) and fused PET/MRI (below right) delineated the tumor. All images courtesy of Dr. Lance Hall and UW Health.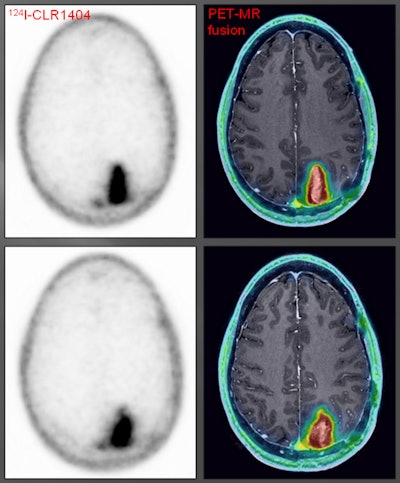
The UW Health researchers are working on a multicenter trial and have begun recruitment to determine if CLR1404 can identify areas of tumor infiltration beyond what MRI can detect.
"Another aspect is whether or not the tracer can differentiate between tumor recurrence and progression in the post-treatment stage," Hall said. "Our hope is that this is something that will provide complementary, additional information to MRI so we can better diagnose tumors before the first round of therapy. Then, in the follow-up stage, [the question is] can we more accurately differentiate tumors from nontumors."
Study disclosures
Jamey Weichert, PhD, is chief scientific officer, technology founder, and director at Cellectar Biosciences. Study co-authors Benjamin Titz and Joseph Grudzinski are also Cellectar Biosciences employees.