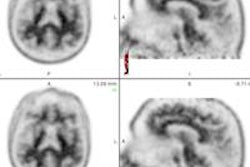
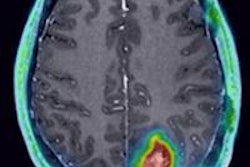
Eli Lilly is touting a study in which florbetapir-PET scans changed the diagnosis and management of patients with Alzheimer's disease.
The study was presented on July 16 at the Alzheimer's Association International Conference (AAIC) in Copenhagen by Dr. Andrew Siderowf, medical director at Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly.
Changes in treatment management were observed based on appropriate use criteria (AUC) developed by the Society of Nuclear Medicine and Molecular Imaging (SNMMI) and the Alzheimer's Association to help determine which patients would benefit by imaging and how best to use the results.
The objective of the study was to evaluate which patients might receive different care if they had an amyloid PET scan as part of their diagnostic workup. In particular, the study evaluated how patients who met appropriateness criteria would be more affected compared to individuals who did not meet AUC standards.
The findings showed that 59% of subjects met the working definition of appropriateness. In addition, 47% of the AUC-like cases were amyloid-positive, compared with 62% of non-AUC cases.
In addition, diagnosis changed after florbetapir-PET scans for 58% of AUC cases, compared with 45% of non-AUC cases. The proportion of patients with a change in management plan was high for both AUC (88%) and non-AUC (77%) cases.
The use of Alzheimer's disease medications also declined after a negative florbetapir-PET scan, from 26 patients to 15 in AUC cases and from 17 patients to eight in non-AUC cases.
Florbetapir is a PET imaging agent that specifically binds to beta amyloid, which has been linked with the onset of Alzheimer's disease and other forms of cognitive impairment. It is marketed under the trade name Amyvid in the U.S. and Europe by Eli Lilly and Avid Radiopharmaceuticals.