GE Healthcare has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Discovery IQ PET/CT system.
First introduced at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting in June, Discovery IQ sports a field-of-view of up to 26 cm and sensitivity of up to 22 cps/kBq.
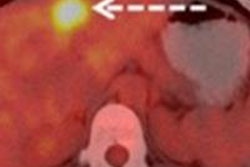
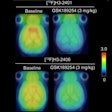
It also includes GE's Q.Clear mode, designed to provide more accurate quantitative measurements for detecting small lesions, with no trade-off between image quality and quantitative measurements of standardized uptake values (SUVs), the company said.