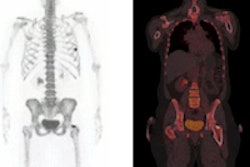
The U.S. Centers for Medicare and Medicaid Services (CMS) has opened a review of its reimbursement policy on sodium fluoride F-18 (NaF-18) PET imaging that could result in an end to the requirement that PET facilities collect data when performing the scans.
CMS opened the review in response to a petition from the National Oncologic PET Registry (NOPR). Imaging centers have been collecting data on the efficacy of NaF-PET as part of CMS' coverage with evidence development (CED) requirement to justify reimbursement for the modality in the management of patient care.
More specifically, Section 220.6.19 of Medicare's National Coverage Determination (NCD) manual established the requirement for prospective data collection under the CED program for NaF-PET for detecting bone metastasis.
CMS is soliciting public comments relevant to the request; in particular, the agency is interested in comments that include scientific evidence and that address the breadth of the request. The petitioners from NOPR include Dr. Bruce Hillner, NOPR chair; Dr. Barry Siegel, NOPR co-chair; and Dr. Anthony Shields, NOPR co-chair.
CMS is taking comments on the petition until April 15. The agency will release its proposal by September 16 and issue a final decision by December 15.