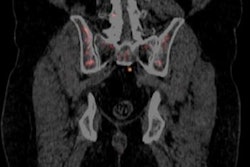
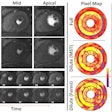
The U.S. Centers for Medicare and Medicaid Services (CMS) has rejected a proposal to loosen the restrictions on reimbursement for sodium fluoride (NaF) PET for detecting cancer that has metastasized to the bone.
On September 16, CMS issued a proposed decision memo stating that it found that NaF-18 PET use was "not reasonable" to identify bone metastases. However, the agency proposed to continue the coverage with evidence development (CED) policy that has been in place for NaF-PET for another 12 months.
CMS implemented the CED structure in 2011, under which it would pay for NaF-PET scans only at sites participating in the National Oncologic PET Registry (NOPR). CMS opened a review of NaF-PET reimbursement in March 2015, after proponents of the modality argued that PET had demonstrated its usefulness enough to justify Medicare payments without the CED restriction.
CMS said it will reconsider its national coverage decision when evidence has been published in a peer-reviewed journal.