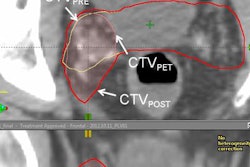
Radiopharmaceutical developer Blue Earth Diagnostics and Siemens Healthineers subsidiary PETNet Solutions said that Blue Earth's Axumin radiopharmaceutical for PET imaging of recurrent prostate cancer will be commercially available this month in the U.S.
Axumin, which received U.S. Food and Drug Administration approval in late May, is indicated for use in PET studies to identify suspected sites of prostate cancer recurrence in men who have elevated blood levels of prostate-specific antigen (PSA) following prior treatment, according to the companies. It contains a fluciclovine amino acid labeled with an F-18 radioisotope.
PETNet serves as Blue Earth's manufacturer and exclusive commercial distributor in the U.S. Initial commercial production of Axumin will soon begin at certain PETNet radiopharmacies, according to the vendors. Blue Earth and Siemens said they are planning on broader availability for the radiopharmaceutical in the coming months.