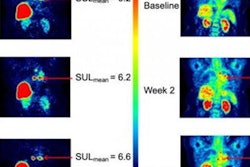
Siemens Healthineers subsidiary PETNet Solutions and Zionexa have announced that the U.S. Food and Drug Administration (FDA) has approved Cerianna (fluoroestradiol) as a radiopharmaceutical for PET scans for recurrent or metastatic breast cancer.
Cerianna is the first FDA-approved F-18 radiopharmaceutical specifically indicated for use in PET detection of estrogen receptor-positive lesions as an adjunct to biopsy in patients with recurrent or metastatic breast cancer, according to the companies.
Cerianna will be commercially available in late 2020 or early 2021 through PETNet Solutions.