CHICAGO - Philips Healthcare is putting a big emphasis on imaging informatics software in its booth at RSNA 2016, rolling out a series of new applications for a variety of modalities and clinical applications.
The new releases range from a package of software and services designed to help imaging facilities improve their operations to an application that brings adaptive intelligence to support radiologists as they interpret medical images.
Imaging informatics
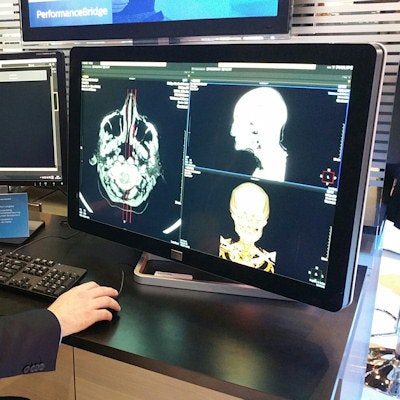
PerformanceBridge is a suite of performance management software and services designed to help radiology departments improve their productivity and deliver better user experience and patient care, while reducing the delivery of unnecessary healthcare. It offers a dashboard-based approach to operational parameters that is designed to help administrators manage their departments from one screen.
PerformanceBridge can run in a cloud-based or on-premise configuration, and it gives department personnel access to data in near real-time by unifying information across different imaging modalities and information systems, pulling data from sources such as PACS and electronic medical record (EMR) networks. The software is able to receive data generated from any vendor's products.
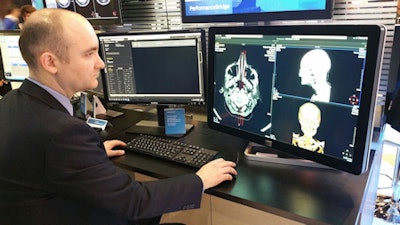 PerformanceBridge is designed to give users an operational dashboard to improve efficiency.
PerformanceBridge is designed to give users an operational dashboard to improve efficiency.Philips is also making dedicated application specialists available, who can help users leverage the tools and insights in PerformanceBridge to improve their departments. PerformanceBridge also includes a radiation dose management function, working in conjunction with the company's DoseWise Portal software for radiation dose management and monitoring.
The company plans to begin rolling out PerformanceBridge in 2017, with volume deliveries beginning in the third quarter. An early adopter of the site is Lahey Hospital and Medical Center in Burlington, MA, which co-developed the software.
Meanwhile, Illumeo marks Philips' entry into what it calls adaptive intelligence, or the combination of artificial intelligence with domain-specific knowledge such as that found in radiology. The software records the preferences of radiologists and adapts its user interface to assist physicians by offering tools and measurements designed to meet the clinical context of the images being interpreted.
Illumeo integrates with Philips' IntelliSpace PACS reading environment, using a data analytics engine to give radiologists the most relevant case-related information from multiple sources, integrated into a single view. For example, this could include the patient problem list, lab results, prior radiology reports, imaging orders, and scanned documents from sources such as handwritten letters, as well as data from EMRs and RIS.
 Illumeo includes tools such as an "inspection mode" that helps users interact with images.
Illumeo includes tools such as an "inspection mode" that helps users interact with images.The software also understands the anatomical context of what the radiologist is looking at on the screen and adjusts its interface accordingly, such as by offering measurement tools if vessels are being analyzed. The software also includes a semantic labeling engine that reads and analyzes image tags and descriptions from multiple vendors, providing automatic semantic labeling and meaningful descriptions.
Radiologists can also generate dynamic reports that include 3D images or quantification of image data. Reports can be integrated with PACS and EMRs and shared as a multidisciplinary patient dashboard across a hospital enterprise to make collaboration easier for a patient's care team.
Philips plans to being shipping Illumeo in mid-2017 as a tool used in conjunction with its IntelliSpace PACS. The company also plans to expand Illumeo's adaptive intelligence concept to other domains.
DoseWise Portal is Philips' radiation dose monitoring and management software, and the company is demonstrating version 2.2 of the software. It now offers integration with IntelliSpace PACS, with users able to open DoseWise from within the PACS application. DoseWise also now accepts data from scanners manufactured by other vendors.
DoseWise is designed to meet the requirements of upcoming rules on radiation set to go into effect in the European Union in February 2018. Philips is reporting that DoseWise users are seeing reductions of 50% to 70% in radiation dose after adopting the technology.
Meanwhile, Philips is giving RSNA attendees a first look at IntelliSpace Universal Data Manager, software for data management that enables enterprises to organize large datasets and deliver them to referring physicians throughout a healthcare network. The software operates similar to a vendor-neutral archive by enabling users to employ different viewers for interpreting the data within.
Finally, IntelliSpace Portal 9.0 is the latest version of the company's visual analysis platform, with multimodality functions and expanded tools for neurological imaging.
MRI
Software is also a highlight in the MRI section of Philips' booth at RSNA 2016. The company is demonstrating new applications for neuroimaging, such as NeuroQuant, a tool for brain imaging that quantifies data acquired from a patient and compares it with a database of normal individuals to assist in diagnosing pathologies such as Alzheimer's disease.
NeuroQuant is designed to monitor disease progression by offering longitudinal comparisons in brain imaging, which is useful for applications such as tracking lesion changes. It could also be used as a tool to enhance doctor-patient communication.
Finally, Philips is demonstrating virtual reality headgear intended to allay patient concerns about MRI scans by giving them a virtual tour of the MRI suite before their real scan.
 Virtual reality headgear gives users a virtual tour of the MRI suite to allay patient concerns.
Virtual reality headgear gives users a virtual tour of the MRI suite to allay patient concerns.CT
In CT, Philips is once again shining the spotlight on its IQon spectral CT scanner. The scanner features "always on" spectral capabilities, according to the vendor. Users don't have to decide whether to turn the spectral mode on; scans can be reconstructed in either spectral or conventional modes. Spectral images can even be reconstructed retrospectively.
Philips has installed 35 IQon scanners so far worldwide.
Molecular imaging
Philips first debuted its Vereos digital PET/CT platform at the RSNA 2015 meeting, and it is again emphasizing the system in its booth.
Vereos replaces photomultiplier-based detector technology with solid-state instrumentation. It also features the vendor's proprietary digital photon-counting technology, which is designed to reduce the tradeoff between sensitivity and resolution. Philips said the digital photon-counting technology yields approximately twice the volumetric resolution, sensitivity gain, and quantitative accuracy of analog systems.
Vereos is scheduled to go into production in the third quarter of 2017, with deliveries expected to begin for new orders in the first quarter of 2018.
X-ray/mammography
Philips is highlighting its CombiDiagnost R90 fluoroscopy system as a work-in-progress. Currently pending U.S. Food and Drug Administration (FDA) 510(k) clearance, the all-digital, remote-controlled fluoroscopy system combines the benefits of radiography and fluoroscopy, according to the vendor. The system, which utilizes both large and small SkyPlate wireless portable digital detectors, also includes the vendor's Unique postprocessing software and dose management features. It can also be configured to serve as full digital radiography (DR) room.
In addition, the company is displaying two mobile radiography offerings: MobileDiagnost wDR and MobileDiagnost M50. MobileDiagnost wDR includes a sliding arm, SkyPlate wireless portable digital detectors, SkyFlow gridless digital x-ray technology, and Unique postprocessing software.
A budget-oriented offering, MobileDiagnost M50 features a small form factor and is suitable for the operating room, emergency room, intensive care unit, and general ward, according to Philips. It also includes the company's Unique postprocessing software.
In mammography developments, Philips is discussing its MicroDose SI full-field digital mammography system, including details on its plans to incorporate digital breast tomosynthesis capabilities.
Image-guided therapy
The company is highlighting its AlluraClarity angiography system with ClarityIQ technology at this year's meeting. ClarityIQ yields equivalent image quality at lower x-ray dose levels for a full range of clinical procedures, a claim that Philips said has been cleared by the FDA.
In addition, Philips is sharing its OncoSuite package of imaging tools for planning, navigating, treating, and following up oncology procedures. OncoSuite also includes EmboGuide for transarterial chemoembolization procedures, the company said.
NeuroSuite is designed to offer enhanced 2D and 3D imaging of intracranial vessels and devices for neuro procedures. It also comes with AneurysmFlow, which aids flow diverter placement in cerebral aneurysms, Philips said.
Philips is also showing its Hybrid OR Suite, a multifunctional imaging system that supports a full range of surgical procedures. It includes the firm's Vessel Navigator software.
Ultrasound
Philips is presenting its Epiq premium system for general imaging with Evolution 3.0 software, as well as Affiniti 30, a new mid-range system with Continuum 1.0 software.
Key features of the systems include MaxVue, a full high-definition display that extends the display area of the Epiq and Affiniti scanners to 21.5 inches.
In addition, the company is devoting attention to its ElastQ Imaging elastography technology. Currently pending FDA 510(k) clearance, ElastQ offers real-time, color-coded shear-wave elastography over a large region of interest, and is designed to help reduce or prevent liver biopsies, according to the firm.
Philips is also highlighting its OmniSphere business intelligence software, which comprises the vendor's Remote Technical Connect and Utilization Optimizer services. Remote Technical Connect supports remote system maintenance, while Utilization Optimizer acquires ultrasound utilization data and organizes the information in a dashboard for Philips ultrasound users.
Philips is also touting its "one-button workflow" for viewing ultrasound contrast agents on its systems and support for ultrasound contrast scanning on many of its transducers.
Finally, Philips is showcasing developments with its Lumify ultrasound platform for Android-based mobile devices. A new transducer -- Lumify S4-1 -- received FDA 510(k) clearance in October and brings new cardiac imaging capabilities to the platform. It's suitable for use in ambulatory care and by home health professionals, according to the firm.




















