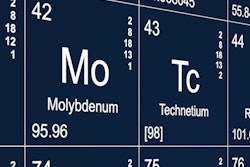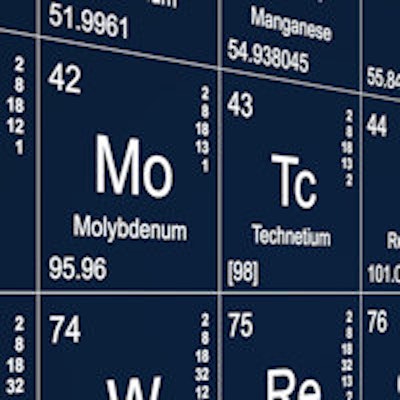
A U.S.-based supply of molybdenum-99 (Mo-99) is finally at hand: The U.S. Food and Drug Administration (FDA) has approved NorthStar Medical Radioisotopes' RadioGenix isotope separation system, and the U.S. Nuclear Regulatory Commission (NRC) will license the system's production of technetium-99m (Tc-99m) for medical use.
RadioGenix has been approved for the production of sodium pertechnetate Tc-99m injection to be injected intravenously, instilled into the bladder or eye, or used with other FDA-approved imaging drugs to examine specific tissues and organs, according to the FDA. The system is the first to use a nonuranium process for producing Mo-99 -- the precursor to Tc-99m, the FDA said. The agency noted that the approval did not require new clinical studies because it relied on safety and efficacy information from an already FDA-approved Tc-99m generator.
Tc-99m is used in more than 80% of nuclear medicine imaging procedures in the U.S. However, Mo-99 could only be previously be produced from enriched uranium by several facilities outside of the U.S., and the complicated supply chain sometimes resulted in shortages of Tc-99m, according to Dr. Janet Woodcock, director of the FDA's Center for Drug Evaluation and Research.
"Today's approval has been the result of years of coordination across the FDA and with U.S. government organizations and marks the first domestic supply of Mo-99 -- the source of Tc-99m -- in 30 years, which will help to ensure more reliable, clean, and secure access to this important imaging agent used in nuclear medicine," Woodcock said in a statement.
In its guidance, the NRC will advise medical and commercial nuclear pharmacy users on the license amendments they will need to possess and use RadioGenix.