Patients who undergo radiation treatment with lutetium-177 (Lu-177)-DOTATATE for neuroendocrine tumors should receive and carry a travel card with details of their regimen to reduce unnecessary security checkpoint delays, according to an article published in the April issue of the Journal of Nuclear Medicine.
The recommendation comes from a group of researchers and patient advocates, some of whom have had the experience of having to spend an hour or longer to clear passage after setting off radiation detectors in airports.
"In our experience, it is not uncommon for patients recently treated with Lu-177 DOTATATE to be stopped by U.S. Customs and Border Protection officers while traveling, especially when crossing the U.S. border at ports of entry, because of the detection of residual radiation activity," wrote the authors, led by Dr. Ayse Tuba Kendi from Mayo Clinic in Rochester, MN. "Detention at the border, even if temporary, can present significant challenges if the patients and their traveling companions are not properly prepared."
Lu-177 DOTATATE (Lutathera, Advanced Accelerator Applications) received clearance from the U.S. Food and Drug Administration (FDA) in 2018, followed with approval in 2019 by Health Canada to treat advanced somatostatin receptor-positive gastroenteropancreatic neuroendocrine (GEP-NET) tumors.
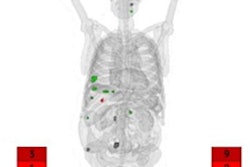
Lutathera is a radiopharmaceutical designed to be injected into patients to deliver radiation directly to tumors. It consists of a radionuclide linked to a peptide that binds to somatostatin receptors on the surface of GEP-NET tumor cells.
Radiation emission after Lu-177-DOTATATE therapy is low, which allows for treatment on an outpatient basis. While residual radiation activity from the treatment is not harmful to others, lingering effects can trigger sensitive radiation detectors at airports and other travel checkpoints (JNM, April 2020, Vol. 61:4, pp. 496-497).
There also is the issue of the metastable isotope mLu-177, which is present after Lu-177-DOTATATE treatment. Lu-177 has a half-life of 6.6 days, but even a small amount of mLu-177 has a half-life of 160 days and can be mistaken for plutonium, since both substances have similar radiation signatures.
"Instrumentation engineers have made considerable improvements in identification algorithms since then, but occasional errors still occur," the authors wrote. "If there is a reading from radiation detectors suggesting plutonium, further review of the data by a trained human analyst is needed for a final decision/clearance. This most likely contributes to the additional waiting time that patients mentioned in the survey."
Travel cards could help identify these individuals when traveling. Kendi and colleagues recommended the cards contain a patient's personal identification, the type of radiation they received, dose of radiation administered, and date of administration.
"Patients need to be provided with a [travel] card that they can carry at all times after each cycle of therapy, detailing the treatment they received," Kendi and colleagues added. "This card should also state that radioactivity can be detected for several weeks after therapy. After the last cycle, patients should carry the last card provided for an additional three months."
While most patients are treated respectfully by security personnel, a public online survey performed by study co-author Josh Mailman found that screening procedures can take one to two hours and longer in some cases. Some respondents reported having to go through the screening process multiple times, as portable detector systems were unable to correctly identify Lu-177 and displayed other radionuclides in the results.
To help avoid these "unwanted surprises," the authors encourage clinical treatment, nuclear medicine, or nuclear radiology personnel to discuss with patients before treatment the possibility of future travel speedbumps and adjust schedules accordingly in advance.