A study by researchers from the U.S. National Cancer Institute (NCI) has shown that hybrid PET/MR imaging exams in pregnant women pose little risk of harmful radiation exposure to the fetus. The results were published online February 3 in the Journal of Nuclear Medicine.
The researchers studied data from women injected with F-18 FDG during PET/MRI exams for cancer staging during the first two trimesters of pregnancy. Fetal radiation doses were very low, they found, and the group recommended that F-18 FDG scans in women with cancer should not be withheld because of pregnancy.
"The benefits for both mother and fetus of a clinically appropriate F-18 FDG-PET scan outweigh the hypothetical risks to the fetus," wrote first author and staff scientist Dr. Paolo Zanotti-Fregonara, PhD, of the NCI's molecular imaging branch, and colleagues.
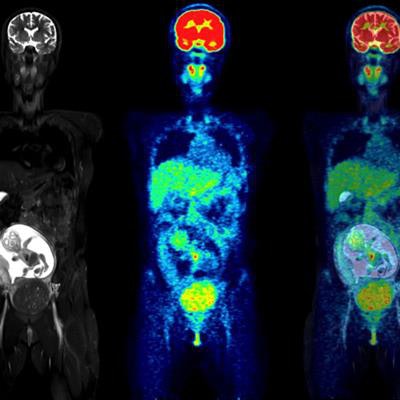
 Coronal slices of MRI (left), PET (center), and fused PET/MRI (right) of a woman at 18 weeks of pregnancy. Of particular interest is the level of uptake in the fetal organs. While the heart showed high F-18 FDG uptake, the brain had only a low level of glucose consumption, especially compared with brain uptake of the mother. This pattern of low glucose consumption has previously been noted even in mature fetuses. All images courtesy of the Journal of Nuclear Medicine.
Coronal slices of MRI (left), PET (center), and fused PET/MRI (right) of a woman at 18 weeks of pregnancy. Of particular interest is the level of uptake in the fetal organs. While the heart showed high F-18 FDG uptake, the brain had only a low level of glucose consumption, especially compared with brain uptake of the mother. This pattern of low glucose consumption has previously been noted even in mature fetuses. All images courtesy of the Journal of Nuclear Medicine.Cervical and ovarian cancers are the most common gynecological cancers diagnosed during pregnancy, and malignancy during pregnancy is increasing, according to the authors. Studies show the most common type of malignancy is uterine cervical cancer.
Radiation exposure to the fetus from F-18 FDG is an essential piece of information when considering PET imaging to stage cancers during pregnancy. To date, evidence has been gleaned only from a few human case reports using either PET alone or hybrid PET/CT scans, which may not accurately identify the soft tissues of the fetus to calculate doses, the authors wrote.
Moreover, most guidelines and expert opinions recommend against the use of PET/CT in pregnancy, due to unknown fetal radiation exposure concerns.
In this study, Zanotti-Fregonara and Japanese colleagues analyzed data from 11 women who underwent F-18 FDG PET/MRI scans (Biograph mMR, Siemens Healthineers) at Fukushima Medical University Hospital for cervical cancer staging during the first two trimesters of pregnancy. F-18 FDG dose estimates were calculated using a voxel-based methodology Zanotti-Fregonara at al proposed in a 2016 study that used anthropomorphic phantoms.
The women were injected with approximately 4 MBq/kg of F-18 FDG (average injected activity, 213 ± 52 MBq) prior to imaging. In part, the fraction of injected activity concentrated by the fetus was derived from manually drawing regions of interest on the MRI slices.
All fetuses were visible in detail on the MRI scans, which allowed the delineation of their body contours. F-18 FDG activity was unevenly distributed in the fetal body. The hearts were generally visible, while the brain showed low uptake, the results showed.
The estimated fetal doses were as follows:
- Early pregnancy: 2.21 E-02 mGy/MBq (n = 1)
- End of first trimester: 7.38 ± 0.25 E-03 mGy/MBq (n = 3)
- During second trimester: 4.92 ± 1.53 E-03 mGy/MBq (n = 8)
"The present dose estimates confirmed the low level of radiation absorbed by the fetus when the mother is injected with F-18 FDG," the authors wrote.
In addition, the highest estimate was observed in early pregnancy (3.2 mGy), while the average of the remaining cases was 1.1 ± 0.5 mGy. The fetus of one woman who had two examinations received a cumulative dose of 3.3 mGy.
These values are more than one order of magnitude lower than the threshold considered high enough to damage living tissues, the authors wrote.
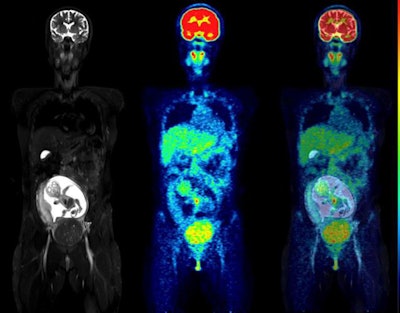
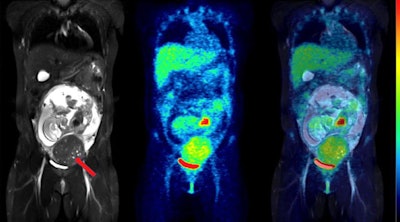 Coronal slices of MRI (left), PET (center), and fused PET/MRI (right) of the same woman as above, but at 24 weeks of pregnancy. The fetus has visibly increased in mass, but the pattern of F-18 FDG uptake in the organs has not changed, with the heart showing high glucose consumption but the brain still largely silent. The red arrow points to a uterine fibroid.
Coronal slices of MRI (left), PET (center), and fused PET/MRI (right) of the same woman as above, but at 24 weeks of pregnancy. The fetus has visibly increased in mass, but the pattern of F-18 FDG uptake in the organs has not changed, with the heart showing high glucose consumption but the brain still largely silent. The red arrow points to a uterine fibroid.This was the first such F-18 FDG dosing study in pregnant women to use imaging entirely acquired with PET/MRI, which allowed detailed visualization of the fetal body contours, they noted. Hence, dose estimations were arguably more accurate than in previous reports, according to the group.
Moreover, such accuracy cannot be achieved by coregistering the PET image to an MRI acquired separately because the fetus would have moved in the meantime.
"If the PET scan of a pregnant woman is planned, we encourage nuclear medicine departments to acquire dynamic images," the authors concluded. "These would not increase the radiation dose, but would allow an even more accurate assessment of fetal dosimetry."