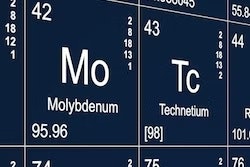
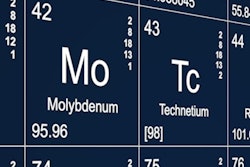
Last year was a bumpy ride for a U.S. initiative to secure domestic supplies of molybdenum-99, according to an official who spoke at a National Nuclear Security Administration (NNSA) meeting October 9 in Arlington, VA.
NNSA representatives and key stakeholders in the nuclear medicine industry met to provide updates on a program launched in 2019 to develop technology and build new facilities to achieve the goal.
“Despite the significant progress on physical infrastructure, U.S. companies have experienced significant challenges with financing and commercialization. A lot of these issues came to a head late last year,” said Max Postman, director of the NNSA’s Office of Reactor Conversion and Uranium Supply.
Mo-99’s daughter decay chain isotope is technetium-99m (Tc-99m), which is used in an estimated 40,000 diagnostic nuclear medicine exams each day.
In February 2019, NNSA launched a program to help fund U.S. companies to supply Mo-99 without using highly enriched uranium (HEU). HEU is a proliferation-sensitive material that, if diverted or stolen, could be used as a component of a nuclear weapon, according to the agency. At the time, the U.S. was exporting HEU to foreign-owned reactors to produce Mo-99.
Postman noted that in 2021, the U.S. ended such exports, citing sufficient supplies of Mo-99 produced without HEU, primarily due to a nuclear research reactor in Belgium converting its Mo-99 production from HEU to high-assay low-enriched uranium (HALEU).
However, despite awards totaling up to approximately $60 million, U.S. companies have yet to reach capacity to satisfy domestic supplies. Postman said the NNSA terminated its cooperative agreement with NorthStar Medical Radioisotopes of Beloit, WI, at the company’s request. It also ended an agreement with Niowave of Lansing, MI, by mutual agreement, Postman said.
Shine Technologies of Janesville, WI, remains the NNSA’s sole remaining cooperative agreement partner. Shine is about 75% complete on a new production facility, the Chrysalis, which is expected to become one of the world’s largest producers of Mo-99.
“NNSA awarded an additional $32 million in funding to that agreement earlier this year. These were funds that were recovered from the terminated agreements with other companies,” Postman said.
Portman also discussed the importance of the U.S. Centers for Medicare and Medicaid Services (CMS) proposed new reimbursement plan in 2025, which includes an add-on payment for hospitals for the use of domestically produced Tc-99m.
The rule would come into effect in 2026 and will help ensure that hospitals that purchase Tc-99m derived from domestically produced Mo-99 are receiving equitable payments compared to hospitals that use Tc-99m from imported Mo-99 that benefits from artificially low prices as a result of foreign government subsidies, he said.
“So this proposed rule is a really important and positive step to address the financial sustainability issues that we have seen over the last year,” Postman said.
Finally, Postman noted the European Commission’s recent approval of a $2.17 billion proposal by the Netherlands to fund construction of a new medical isotope production facility in Petten. The approval came with conditions that the Netherlands implement full cost recovery pricing for the isotopes made at the facility, with verification by an independent trustee, he said.
“This goal of verifiable full cost recovery pricing is something that the international community has been pursuing since at least 2010,” he said.