Utter the word "steroid" around an athlete, and chances are his or her reaction will be less than sporting. The antidoping crusade, which heated up with an investigation of supplement company Bay Area Laboratory Co-Op (BALCO) of South San Francisco, CA, in 2004 has resulted in some athletes being publicly questioned, including Olympic track and field superstar Marion Jones.
Just this week at the Olympic Games in Athens, Russian shot-putter Irina Korzhanenko was stripped of her gold medal after testing positive -- twice -- for the steroid stanozolol.
Earlier this summer, U.S. sprinter Kellie White was suspended for one year from competition after admitting to a regimen of banned drugs included steroids, while another U.S. sprinter, Calvin Harrison, was given a two-year suspension for his second doping violation. Two years ago, British pole-vaulter Janine Whitlock was booted from the Olympics for life after testing positive for anabolic steroids.
But outside the realm of performance enhancement, steroids are a valuable medical treatment. Different types of steroids, including corticosteroids, are routinely prescribed to patients for various ailments ranging from allergies to rheumatoid arthritis. Testosterone-based anabolic steroids -- the enhancing supplement of choice for athletes -- were originally created to treat certain types of anemia and kidney disease in men.
For athletes, anti-inflammatory agents can be beneficial. Most recently, South African researchers looked at whether a single corticosteroid shot could help relieve iliotibial band friction syndrome (ITBFS) in runners. They found that runners who received a corticosteroid injection (versus a placebo) reported less pain two weeks later (British Journal of Sports Medicine, June 2004, Vol. 38:3, pp. 269-272).
Legality, ethics, and sportsmanship aside, the use of steroids, especially over the long term, can have devastating effects on the body. One of the most serious is osteonecrosis or avascular necrosis. A lack of blood supply to an affected area leads to tiny breaks, and ultimately the collapse of the bone from within. It generally involves more than one joint at a time. The most common areas affected are the hips, knees, and shoulders ("Osteonecrosis," University of Washington Orthopaedic & Sports Medicine, September 5, 2001).
Focusing specifically on the hip, osteonecrosis of the femoral head (ONFH) occurs most often between the ages of 30 and 50, and is slightly more frequent in men than in women. The main causes of ONFH are sickle cell disease, chronic alcohol abuse, and long-term steroid use, according to Dr. Philippe Hernigou of the University Paris XII, Hôpital Henri Mondor in Creteil, France (Maîtrise Orthopedique).
MRI has proven to be the go-to modality for pinpointing ONFH. Japanese investigators successfully used MRI for tracking multiple sites of steroid-related osteonecrosis, as well as clarifying the role of bone marrow edema in the early development of the disease. They've also suggested ways to refine MR of the femoral head with more sophisticated imaging techniques. Finally, another group from Japan has made progress in quantifying the connection between steroid dosage and osteonecrosis.
Signs on MRI
A normal femoral head on a T1- and T2-weighted MRI will display with uniform high signal intensity; osteonecrosis appears as a basic pattern of decreased signal intensity.
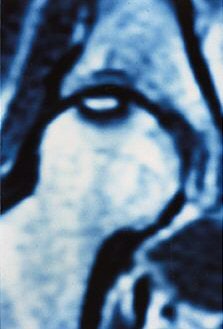 |
| MRI pattern of small, incipient osteonecrosis of the femoral head lesion, anteroposterior and lateral views. The zone inside the necrotic segment is still of high intensity. Images courtesy of Maîtrise Orthopedique and Dr. Philippe Hernigou. |
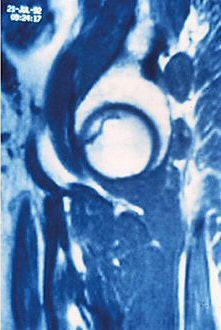 |
"The low-intensity zone may be homogenous or heterogeneous, with a speckling of high intensity against the low-intensity background," Hernigou wrote in the article for Maîtrise Orthopedique. "The most typical image is a thin low-intensity band on T1- and T2-weighted (MRI) that goes to the subchondral bone, and is more or less concave toward the top of the femoral head."
The standard protocol for imaging osteonecrosis includes a T1-weighted axial localizer, T2-weighted spin-echo sequences, or fast T2 gradient-echo sequences. The marrow fat signal can be suppressed with STIR sequences.
Multiple osteonecrosis sites
Orthopedic surgeons in Japan used MRI to screen for multiple osteonecrotic sites in patients diagnosed with ONFH. Specifically, they looked for disease involving two or more anatomic sites.
"Steroid-related osteonecrosis ... tended to develop in multiple joints, whereas idiopathic osteonecrosis did not develop as multiple lesions," wrote Dr. Takashi Sakai in a poster presentation at the 2004 American Academy of Orthopaedic Surgeons (AAOS) conference in San Francisco. Sakai is from the Osaka National Hospital in Suita; his co-authors are from the Osaka University Medical School, also in Suita.
They came to their conclusions after studying 200 patients (104 female, 96 male) who underwent MRI on a 1-tesla Signa scanner (GE Healthcare, Waukesha, WI). The imaging protocol included spin-echo gradient sequences, TR of 14 msec, TE of 2.3 msec, and 1.5-mm slice thickness.
"Normal fat intensity area surrounded by a low-intensity band or diffuse low-intensity area in each site on T1-weighted images or SPGR was defined as osteonecrosis," the authors said.
According to the results, osteonecrosis affected bilateral hips in 125 out of 151 steroid-related patients (83%). Multiple osteonecrosis was found in 107 patients (54%), including 92 steroid-induced cases. Sixty-one percent of these cases were deemed to be steroid-induced. The knee was the most common site for additional osteonecrosis, followed by the shoulder.
"Between multiple osteonecrosis patients and nonmultiple osteonecrosis patients, there were significant differences in age at diagnosis, gender, and related factors. Multiple osteonecrosis tended to occur in steroid-related, young female patients," the authors wrote, adding that the collapse rate for the knee was 27%, 25% for the shoulder, and 17% for the ankle.
3D SPGR
In related research, Sakai and colleagues also reported success with 3D spoiled gradient-echo (3D SPGR) MRI for measuring lesions in ONFH.
"Early and reliable assessment of the size and location of necrotic lesions before progression to collapse is important," wrote lead author Dr. Yuki Kishida. "With the use of 3D SPGR imaging, there is no need for imaging in several orientations at once. Imaging time is shortened, and the burden on the patient is reduced" (Journal of Orthopaedic Research, September 2003, Vol. 21:5, pp. 850-880).
For this study 20 femoral heads were removed postarthroplasty from ONFH patients. Imaging was done on a 1.5-tesla Signa MR/i HiSpeed scanner (GE Healthcare) using T1-weighted SE and 3D SPGR with extremity coils. T1-weighted SE images also were obtained.
In all 20 cases, there was a band of low signal intensity on 3D SPGR images, which correlated with histological evaluation. This band of low signal intensity corresponded to repaired marrow with viable fibrous mesenchymal tissue, according to the authors.
"On 3D SPGR MR imaging, the necrotic was identified as the area proximal to the boundary zone of low signal intensity," they explained. "Sensitivity for the detection of ONFH with both 3D SPGR and T1-weighted SE images was 100% and specificity was 100%." However, the authors did note that the margins of the femoral head were more clearly defined on 3D SPGR.
Bone marrow edema
Sakai and co-authors also reported that 3D SPGR MRI had 100% sensitivity and specificity for ONFH with bone marrow edema. The latter is a controversial issue in ONFH imaging -- can bone marrow edema seen on MR be interpreted as a harbinger of osteonecrosis?
Another group from Japan, led by Dr. Satoshi Iida, looked at the correlation between bone marrow edema and the collapse of the femoral head in steroid-induced osteonecrosis. Orthopedic surgeon Iida is from Chiba University School of Medicine in Chiba City. His co-authors included Dr. Hiroshi Kitahara from the university's radiology department.
"We performed a prospective study using MR imaging in high-risk patients who started receiving high-dose steroids," they wrote. "The purpose of this study ... was to clarify the relation of bone marrow edema to (steroid-induced) osteonecrosis,"(American Journal of Roentgenology, March 2000, Vol. 174:3, pp. 735-743).
One hundred patients (200 hips) were enrolled in an MR screening program. The initial steroid dose was at least 60 mg of prednisolone per day. Eventually, the study population consisted of 46 hips in 26 patients. An initial MR was performed within six months of steroid therapy. Screenings were repeated every one to three months for about one year, then every three to six months afterward. Imaging was done on either an MRT-50 (Toshiba Medical Systems, Tokyo) or a Signa scanner (GE Healthcare). The diagnosis of osteonecrosis on MR was based on band-like abnormal signals and band-like hypointense zones on T1-weighted imaging.
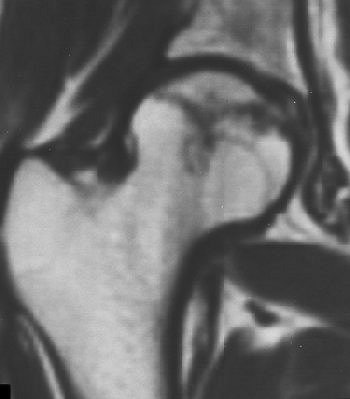 |
| Right femoral head of 24-year-old woman who received high-dose steroid therapy for systemic lupus erythematosus. Coronal T1-weighted MR image (A) obtained 10 weeks after initiation of therapy shows band-like hypointense zone. Coronal STIR image shows matching hyperintense zones (B). Iida S, Harada Y, Shimizu K, Sakamoto M, Ikenoue S, Akita T, Kitahara H, Moriya H, "Correlation Between Bone Marrow Edema and Collapse of the Femoral Head," (AJR 2000; 174: 735-743). |
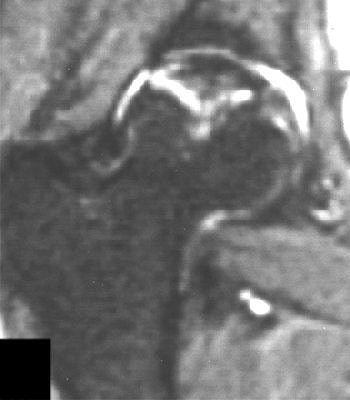 |
According to the results, 28% of the 46 hips had diffuse signal abnormalities in the marrow of the femoral head and neck. On T1-weighted images, these regions were ill-defined and decreased in signal intensity; on STIR images, they were diffusely hyperintense indicating bone marrow edema.
"Bone marrow edema was found in the living bone outside the band-like hypointense zone on the initial MR," the group stated. "At the time bone marrow edema was detectable on MR imaging, all 13 hips were symptomatic."
At final examination, 85% of these cases had progressed to advanced osteonecrosis, and bone marrow edema was highly correlated with the subsequent collapse of the femoral head.
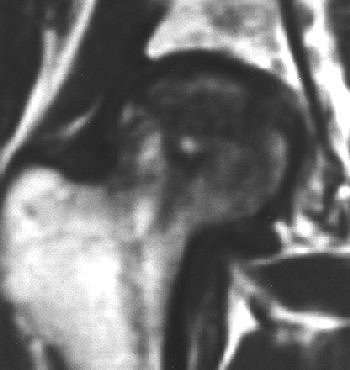 |
| Coronal T1-weighted MR image, obtained six months after A-B and one week after onset of hip pain, shows diffusely decreased signal intensity (D). T1-weighted fat-saturated contrast-enhanced image shows nonenhancing lesion in subchondral area of femoral head surrounded by brightly enhanced marrow in the head and neck (G). Iida S, Harada Y, Shimizu K, Sakamoto M, Ikenoue S, Akita T, Kitahara H, Moriya H, "Correlation Between Bone Marrow Edema and Collapse of the Femoral Head," (AJR 2000, 174: 735-743). |
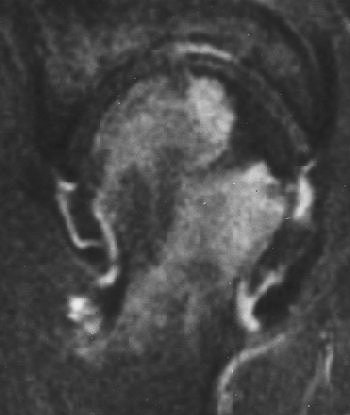 |
Iida's group explained that bone marrow edema should be considered a marker for a progressive illness. Its presence can delineate transient osteonecrosis -- a self-limited disease that can be resolved with treatment -- and osteonecrosis, which can progress to destructive arthrosis.
The 'magic' dose?
While the organizations that rule the sports world actively campaign against steroid use, many athletes still swear by them. There is much debate as to whether anabolic steroids really do work for performance, and to what degree. By design, the results of various scientific inquiries into the effects of steroids may not tell the whole story.
"The use of anabolic steroids in the 'real world' is considerably different from that in rigidly controlled, double-blind experiments," wrote Dr. Thomas Fahey, from the Exercise Physiology Laboratory at the California State University in Chico. "Most studies have not used the same drug dosage used by athletes. Institutional safeguards prohibit administration of high dosages of possibly dangerous substances to human subjects" (Sportscience, March 7, 1998).
Defenders of anabolic steroids claim that the substance itself isn't harmful, but that the danger lies in the dosage. What constitutes a massive overdose? The athletes themselves certainly aren't telling -- reports vary wildly, with some people doping with as little as 100-200 mg of a steroid cocktail per week, and others allegedly taking on hundreds of times the recommend medical dose.
What is known at this point is that higher doses of steroids can make someone more susceptible to osteonecrosis. A multi-institutional group from Japan's Kyoto Prefectural University and the Nagoya University School of Medicine came to this conclusion after measuring the relationship between steroid dosage and osteonecrosis with MRI.
They looked at 45 patients who had undergone renal transplantation and were on a postoperative regimen that included significant doses of oral steroids. They underwent MR exams every six to nine weeks after the transplantation (Transplantation Proceedings, November 1998, Vol. 30:7, pp. 3039-3040).
According to their results, in the 12 patients who developed ONFH, the mean dose of oral steroids was 1,232 mg during the first month and 1,936 mg during the first and second months. The mean dose of intravenous methylprednisolone in these patients was 1,687 mg during the two months.
In comparison, the 33 patients without ONFH had a mean oral steroid dosage of 1,194 mg in the first month and 1,870 mg up to two months. The mean dose of IV methylprednisolone was 1,186 mg. The frequency of ONF was 26.7%.
"These findings ... show that evaluation of steroid dosage up to two months ... is meaningful," wrote Dr. Toshikazu Kubo, Ph.D., and colleagues. "The role of steroids in (ONFH) has not been fully elucidated. Our findings suggest that large doses of methylprednisolone ... might relate to the occurrence of osteonecrosis of the femoral head."
By Shalmali Pal
AuntMinnie.com staff writer
August 26, 2004
Related Reading
Pulsed corticosteroids tied to notable bone loss, August 2, 2004
Many top cyclists have enlarged hearts, July 15, 2004
Cardiac effects of anabolic steroids persist for years, May 23, 2004
Imaging documents damage associated with NSAIDs usage, November 27, 2003
Ultrasound detects occult scaphoid fractures, June 26, 2002
Copyright © 2004 AuntMinnie.com