BERLIN - Mitral valve insufficiency is a fairly common heart problem that lacks ideal imaging solutions. A mitral valve that fails to close properly during the cardiac cycle causes regurgitant blood flow in systole that requires surgical repair or even valve replacement when it becomes serious.
The problem lies in quantifying and evaluating this retrograde flow to determine whether the need for intervention is urgent. Even in mild cases, periodic assessment is needed. But the two imaging methods currently used to assess the retrograde flow, color Doppler ultrasound and phase-contrast MRI, both have limitations.
In a presentation Thursday at the Computer Assisted Radiology and Surgery (CARS) 2005 meeting, Roland Unterhinninghofen from the University of Karlsruhe in Germany discussed the current limitations in blood-flow assessment, and offered a new approach to quantifying retrograde blood flow using time-resolved MRI vector fields.
The project was undertaken in cooperation with the University Hospital of Mainz, Germany, and the German Cancer Research Center in Heidelberg.
"Color Doppler ultrasound ... has some disadvantages such as limited visibility, as the sound beam is often obstructed by bone," Unterhinninghofen explained. "Also, the velocity measurement, which is the basis for the flow quantification, is limited to one dimension, which may lead to an underestimation of the (blood) volume. And finally, ultrasound is prone to turbulence."
As for phase-contrast MRI, it is generally used to quantify flow in large blood vessels, but not the heart itself, Unterhinninghofen said. And like ultrasound, phase-contrast MRI's velocity measurement is unidimensional, and while it is quite accurate at the position where the data is acquired, interactivity is limited: the clinician cannot move the plane measurement during acquisition.
A solution to these limitations may lie in the acquisition of a complete 3D velocity field, where arbitrary flow planes can be determined after the data is acquired to evaluate the data interactively, and obtain a thorough assessment of the flow dynamics, he said. It will also be necessary to segment the flow to quantify it.
"We tried to evaluate an MRI sequence that allows the acquisition of a vector or velocity field of the flow, and we tested it by quantifying flow in large vessels," Unterhinninghofen said.
The large vessels were found in six pigs, which underwent MRI after mitral valve insufficiency was induced surgically. The team used a recently developed phase-contrast cardiac-MRI sequence that allows taking four images per slice and time frame, including one morphological or magnitude image, and three velocity-encoded, or phase, images.
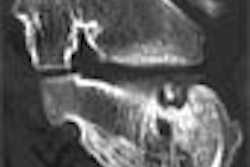
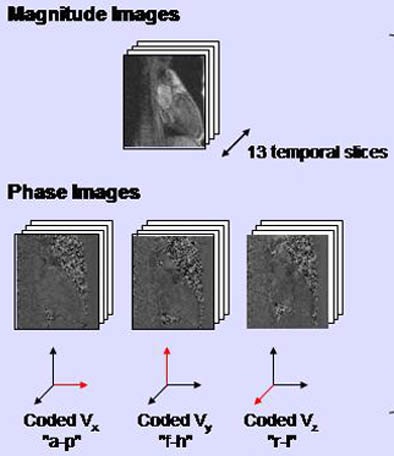 |
| A specially adapted phase-contrast MRI sequence enabled the acquisition of four images per slice and time frame, including one morphological (magnitude) image and three velocity-encoded (phase) images, one for each direction. All images courtesy of Roland Unterhinninghofen. |
The image matrix was 160 x 256 with a spatial resolution of 1.4 x 1.4 mm; 3 x 13 phase images were acquired throughout the cardiac cycle using ECG triggering. The sequence was repeated step by step approximately 24 times for each subject by moving the slice about 2 mm each time.
This produced for each subject a 4D dataset of the morphology, and another 4D dataset of the velocity vectors -- "that is, a time-resolved 3D velocity or vector field," Unterhinninghofen said. He noted that the velocity-encoded images that code the three phases of the velocity vector are not actually acquired at precisely the same time, but rather after delays of a few milliseconds between each phase, potentially inducing measurement errors.
The group developed the MediFrame computing environment to evaluate the data. Using the blood-flow analysis plug-in, the application enables the user to view the beating heart in a 3D ray-traced representation. The display is designed to simulate blood-flow evaluation with color Doppler ultrasound. Thus, the velocity data is colored red and blue using the system's "virtual transducer," and the user simply moves the mouse to choose each new measurement plane.
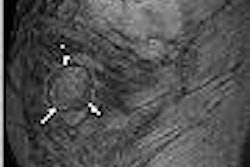
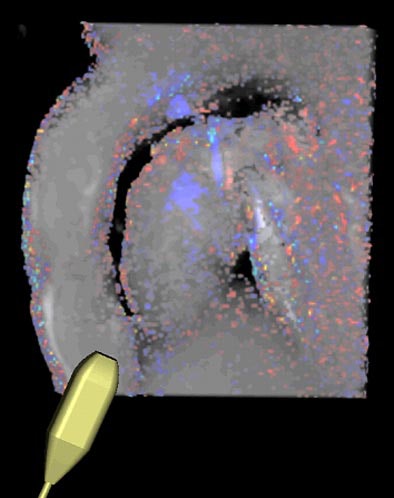 |
| The MediFrame application provides visual assessment of blood-flow velocity in a user interface that mimics a transducer (controlled by a mouse) in the setting of color Doppler ultrasound. |
"Flow quantification is relatively simple, it is just calculating the normal component of the vectors regarding the measurement plane, and then summing up the values," Unterhinninghofen said.
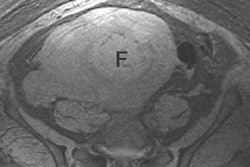
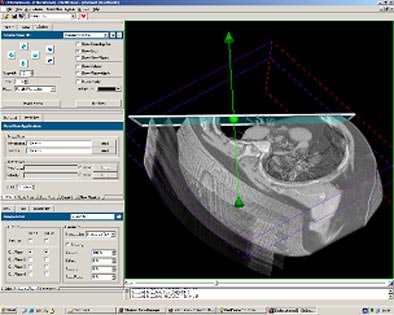 |
| The MediFrame application enables automated blood-flow velocity measurements in multiple planes from 4D (time-resolved 3D) MRI data. |
To test the results, the researchers knew that the blood flow data should be the same for any arbitrary measurement position. So for each dataset they placed the measurement plane at 10 arbitrarily chosen points along the ascending aorta so that it cut through the vessel orthogonally. The size of each plane was adjusted to fit the vessel area as needed, and the plane was systematically rotated in an arbitrary diameter axis to acquire 50 measurements per dataset, Unterhinninghofen said.
According to the results, "the dependency of the measurement on the angle was very low (5% or less), but the dependency on the position is larger than we had expected," he said. So while flow can be expected to decrease as the position moves downward, it did not do so reliably.
Part of the discrepancy may have been due to reduced flow in smaller vessels, but the source of the rest of the error is unclear, and may be due to artifacts or other disturbances in the data, Unterhinninghofen said. And the relatively poor image quality makes it difficult to define the elliptical plane correctly.
"We have built an integrated tool for flow quantification from MRI vector fields, and we have seen that the velocity field is consistent in itself, but what we do not have in this evaluation is a reference measurement," Unterhinninghofen said.
Direct ultrasound measurements will be used as a reference standard in the next phase of the study, and flow segmentation will also be introduced to make the system less prone to position-related errors, he said.
By Eric Barnes
AuntMinnie.com staff writer
June 24, 2005
Related Reading
3D: Rendering a new era, May 2, 2005
MDCT maybe equivalent to MRI, tops echo and SPECT for heart function, February 15, 2005
Copyright © 2005 AuntMinnie.com