Tuesday, November 27 | 11:10 a.m.-11:20 a.m. | SSG01-05 | Arie Crown Theater
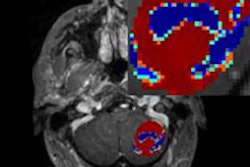
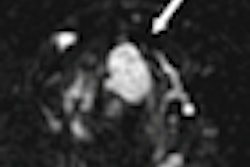
Both dynamic contrast-enhanced (DCE) and diffusion-weighted (DWI) MRI can accurately reflect response to neoadjuvant chemotherapy among breast cancer patients during treatment with minimum apparent diffusion coefficient (ADC) as the most predictive marker of pathologic response.Those conclusions come from a study by researchers at the University of Washington and Seattle Cancer Care Alliance who sought to assess response to neoadjuvant chemotherapy among patients with locally advanced breast cancer.
"Accurate early assessment of neoadjuvant chemotherapy treatment response is imperative as we move toward individualizing therapies," said lead study author Dr. Sana Parsian, a research trainee in the department of radiology. "Early recognition of neoadjuvant chemotherapy nonresponders would allow for timely optimization of therapy protocols that spare patients the toxicity of ineffective agents and can facilitate the identification of tumor adaptation and resistance."
The retrospective study included 21 consecutive patients from January 2006 to May 2009 who received both DCE and DWI-MRI at three time points. All 21 patients were imaged prior to treatment, while 15 were imaged again after 12 weeks of neoadjuvant chemotherapy, and 18 were imaged at the end of therapy.
Before treatment, the mean lesion size was 59.1 ± 27.4 mm at the 12-week mark. At the midpoint of neoadjuvant chemotherapy, changes in peak enhancement and minimum ADC were significantly predictive of complete pathologic response, and change in tumor volume was marginally predictive.
"Our findings show promise for quantitative MRI measures to be used as predictive markers for breast cancer response to chemotherapy, although larger prospective studies are needed to validate our findings," Parsian added.
She and her colleagues are conducting larger prospective studies to further evaluate the predictive value of quantitative dynamic contrast-enhanced and diffusion-weighted MRI characteristics for monitoring response to therapy.
These studies include a project funded by the U.S. National Institutes of Health that is under way to examine associations of breast tumor perfusion and metabolism with treatment resistance, as well as the multi-institutional Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging and Molecular Analysis 2 (I-SPY 2)/American College of Radiology Imaging Network (ACRIN) 6698 trial, using MRI characteristics to both guide treatment and predict outcomes.