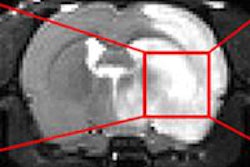
Researchers from Johns Hopkins University have developed new MRI software that may help predict and reduce the risk of complications from clot-dissolving drugs used to treat stroke, according to a study published online December 20 in PLOS One.
The software is designed to overlay MR images with calculations that more precisely measure blood-brain barrier damage in stroke victims. It could lead to safer, more individualized treatment of blood clots and better patient outcomes.
The blood-brain barrier shields blood vessels that limit the passage of molecules from the bloodstream into the brain. Without this barrier, the brain is open to infection, inflammation, and hemorrhage.
Lead study author Dr. Richard Leigh, an assistant professor of neurology and radiology, said a better characterization of damage to the blood-brain barrier could result in new approaches to treating stroke patients.
When patients come to a hospital within three to four hours of suffering an ischemic stroke, doctors often give them the clot-busting drug tissue plasminogen activator (tPA) to dissolve the clot without causing additional damage.
In approximately 6% of stroke patients treated in this manner, too much damage has already been done to the blood-brain barrier, and tPA can cause bleeding in the brain, severe injury, and sometimes death. The problem is that doctors currently don't know which patients could experience this bad outcome.
If physicians knew the extent of the damage to the blood-brain barrier, Leigh said, they could choose a potentially safer treatment option.
For stroke patients who do not arrive at a hospital within the three- to four-hour window for optimal tPA use, physicians believe it is dangerous to give the clot buster for fear of hemorrhage. More aggressive treatment, such as removing the clot with a catheter threaded from the groin or by directly injecting tPA into the brain, may be a better option for these patients, Leigh added.
Before treatment, these patients traditionally have received an MRI exam. However, there is no reliable way to detect the subtle amount of blood-brain barrier damage that could help determine which treatment would be best for a patient, the study authors noted.
The Johns Hopkins software uses MR images and overlays them with calculations that more precisely measure blood-brain barrier damage. The use of the software could show that tPA would be safely used even if stroke patients arrive at the hospital later than safe-use guidelines indicate.
The software would allow physicians to evaluate patients on a case-by-case basis to determine which individuals would benefit by which treatment, Leigh said. In addition, the number of stroke-related complications may also be reduced.
Leigh and his colleagues recommend more research before the software can be widely used. The researchers are looking to assemble a larger patient cohort to better define how physicians can use this information to choose the most appropriate treatment.