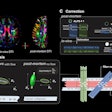
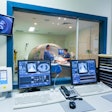
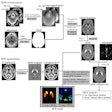
Focused-ultrasound developer InSightec has treated the first patient for essential tremor in a phase III clinical trial of its ExAblate Neuro system.
The multicenter, double-blind, randomized trial will evaluate the efficacy and safety of treatment using ExAblate Neuro in medication-refractory essential-tremor patients, according to the Israeli firm.
The study will enroll 72 patients in up to eight centers around the world. The patients will be randomized to receive either ExAblate Neuro or placebo, and they will be assessed at six and 12 months. They will also be followed, as directed by their doctor, for up to five years.
InSightec expects that the trial results will support a premarket approval (PMA) submission to the U.S. Food and Drug Administration (FDA) for ExAblate Neuro.
The U.S.-Israel Binational Industry R&D (BIRD) Foundation, the Focused Ultrasound Foundation, and InSightec are partnering to support this clinical trial, InSightec said.


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)


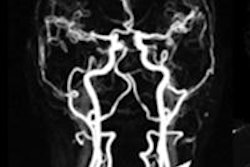

.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)