Multiparametric 1.5-tesla MRI scans can be performed safely for certain prostate cancer patients with cardiac implantable electronic devices (CIEDs), but precautions are warranted, according to a study published in the April issue of the American Journal of Roentgenology.
Under controlled conditions and optimum MRI parameters, acceptable image quality was achieved when scanning patients with cardiac implants, and no patients experienced changes in heart rates or rhythms or felt any heating sensations from the 1.5-tesla magnet, even with the addition of an endorectal coil.
"No serious MRI-related adverse events were observed in our study," wrote lead author Dr. Takashi Tanaka and colleagues from three Mayo Clinic campuses. "However, systematic safety precautions, including careful patient selection according to the risk-to-benefit ratio, consideration of an alternative imaging modality, and the clinical necessity of MRI, appropriate device reprogramming, and close patient monitoring during and after MRI examinations are required."
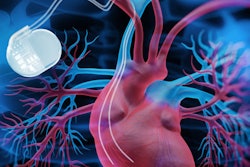
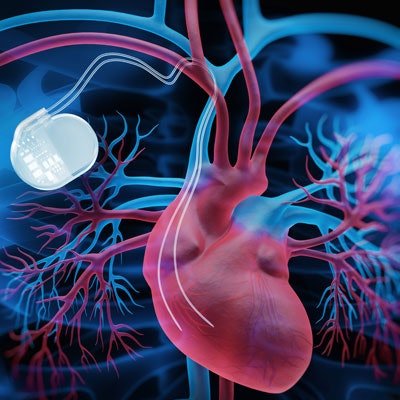
More than 2 million people in the U.S. have CIEDs, such as pacemakers or implantable cardioverter-defibrillators (ICDs). It is estimated that between 50% and 70% of them will have an MRI scan during their lifetime. While an implanted CIED can be a contraindication to MRI, MRI-conditional devices make it possible for some of these patients to undergo a 1.5-tesla MRI exam of the prostate with minimal risk.
How can clinicians eliminate risk during MRI scanning, even if they don't know whether the patient has an implant with MRI-conditional status? And how can they achieve a balance between patient safety and diagnostic-quality images?
"To our knowledge, the safety and quality of prostate multiparametric MRI in patients with a conventional or MRI-conditional CIED have not been reported," the authors noted. "The purpose of this study was to determine the safety and image quality of 1.5-tesla prostate multiparametric MRI with an endorectal coil for evaluation of patients with a CIED."
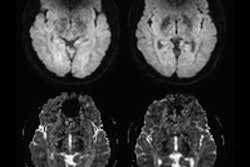
For this retrospective study, Tanaka and colleagues searched records at two Mayo Clinic facilities between January 2012 and June 2016 and found 25 patients who met the inclusion criteria. The team examined 28 prostate scans taken on 1.5-tesla MRI systems (Optima MR450w or Signa HDxt, GE Healthcare) with an endorectal coil and pelvic phased-array coil. All subjects had either an implanted pacemaker or ICD and were matched with patients with no CIEDs, who served as a control group (AJR, April 2019, Vol. 212:4, pp. 815-822).
The study also included two independent readers who rated image quality and artifacts on a five-point scale based on T2-weighted, diffusion-weighted, and dynamic contrast-enhanced images.
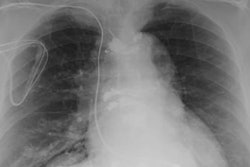
The researchers found no serious adverse physical or CIED-related effects, such as irregular heartbeat or rhythm, from the 1.5-tesla scans with the coils; no scan was halted before its completion; and no other complications occurred. The clinicians took several precautions for the scans, including regulating the specific absorption rate (SAR), which indicates the amount of energy emitted by an MRI scanner and absorbed by a patient, to less than 1.5 to 2.0 watts per kilogram. By doing so, they minimized the risk of CIED leads heating and causing thermal injury.
As for image quality, the team found no statistically significant difference between the two readers based on regional anatomy, artifacts, and overall imaging preferences in either the CIED group or control subjects. Their lack of differentiation also held true for the three MR imaging sequences individually and when combined.
"In this study, patients with a CIED could safely undergo prostate 1.5-tesla multiparametric MRI under controlled conditions with acceptable diagnostic image quality using a low-SAR protocol," the researchers wrote.