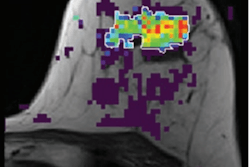
A comparison of two gadolinium-based contrast agents (GBCAs) for hepatobiliary MRI shows that both agents can cause respiratory and motion issues for patients, but the issues were more pronounced with one of the agents, according to a study to be published in the November issue of Radiology.
U.S and Swiss researchers found that approximately three-quarters of patients experienced respiratory issues with gadoxetate disodium (Eovist, Bayer HealthCare), compared with gadoterate meglumine (Dotarem, Guerbet), and the motion irregularities lasted significantly longer.
"Our primary goal was to assess whether the administration of various types of gadolinium-based contrast agents potentially leads to respiratory irregularities during free breathing and whether these irregularities differed in intensity," wrote the authors, led by Dr. Carl Glessgen, from University Hospital Basel (Radiology, November 2019, Vol. 293:2, pp. 317-326). "Our primary hypothesis can be accepted because we observed substantial respiratory irregularities leading to a substantial liver translation in both study groups."
Over the past 15 years, gadoxetate disodium has become an important diagnostic tool for hepatobiliary MRI for the liver and gallbladder, in part because the GBCA is known to leave the patient's system faster than other comparable gadolinium-based agents. While gadoxetate disodium has accumulated an excellent safety record over that time, it also is known to cause short-term respiratory issues, such as labored breathing during arterial MR imaging phases. Some previous research has explored GBCA-related respiratory motion, but few studies have analyzed respiratory changes caused by gadoxetate disodium or how other GBCAs fare in comparison.
To investigate the question, Glessgen and colleagues prospectively recruited 497 consecutive patients scheduled to undergo abdominal free-breathing MRI scans on either a 1.5- or 3.0-tesla MRI system (Magnetom Avanto or Magnetom Skyra, Siemens Healthineers) between January 2015 and May 2017. The cohort included 338 patients (mean age, 59 ± 15 years) who underwent MR imaging with gadoxetate disodium for focal liver lesions or suspected cirrhosis and 159 participants (mean age, 59 ± 17 years) who received gadoterate meglumine for their scans.
The protocol included a golden-angle radial sparse parallel (GRASP) sequence that combines compressed sensing, parallel imaging, and golden-angle radial sampling. The MRI exams produced 497 image datasets, from which aortic and hepatic assessments were performed at an interval of 10-second imaging points.
The choice of GBCA was based, in part, on the type of tumor clinicians were investigating. For example, gadoxetate disodium was used for primary hepatic or metastatic tumors, while gadoterate meglumine was commonly used for suspected vascular tumors.
In comparing the two agents, the researchers found that both GBCAs prompted substantial hepatic motion due to respiratory issues, but the agents differed significantly in a few key criteria:
- An arterial dose of gadoxetate disodium (19.7 sec) arrived significantly later than gadoterate meglumine (16.3 sec) (p < 0.001).
- Hepatic respiratory motion occurred in more patients (239 patients, 71%) who received gadoxetate disodium than patients (46 patients, 29%) who were given gadoterate meglumine (p < 0.001).
- The duration of the motion irregularities was longer in the gadoxetate disodium group (19.2 sec), compared with gadoterate meglumine patients (17.2 sec) (p < 0.001).
Glessgen and colleagues noted that several factors could contribute to the breathing irregularities after GBCA administration. For example, patients with cirrhosis often have pleural effusion, which can cause breathing problems.
They concluded, however, that the administration of the two different gadolinium-based contrast agents at free-breathing conditions potentially may lead to respiratory issues with differing intensity and onset.