Polarean Imaging has submitted a new drug application (NDA) to the U.S. Food and Drug Administration (FDA) for its drug-device combination that uses hyperpolarized xenon-129 (Xe-129) gas with MRI to visualize and quantify regional lung function.
Polarean is requesting priority review from the FDA following the completion of two phase III clinical trials for the drug-device combination.
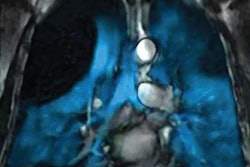
Xe-129 enables functional, regional, and quantitative MR imaging of the lungs when polarized with the system. Patients inhale Xe-129 as a gas during a 10-second breath-hold MRI scan. In the clinical trials, ventilation in zones of interest were quantified and compared with from a different imaging modality that were similarly quantified.