Researchers have found that MRI helps evaluate the effects of Alzheimer's treatment with the drug aducanumab -- sold commercially as Aduhelm -- and adjust or suspend dosage if needed, according to a study published November 22 in JAMA Neurology.
Aducanumab was developed by Biogen for the treatment of Alzheimer's disease. The drug was approved by the U.S. Food and Drug Administration in June 2021 under an accelerated regulatory pathway, a move that was controversial both due to the cost of Aduhelm as well as the equivocal nature of the data supporting the drug's effectiveness.
In the current study, aducanumab can actually cause amyloid-related imaging abnormalities in patients with mild cognitive impairment or dementia due to Alzheimer's disease, wrote a team led by Dr. Stephen Salloway of Brown University in Providence, RI.
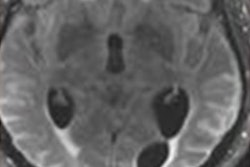
"Amyloid-related imaging abnormalities (ARIA) comprise a spectrum of imaging findings detected on brain MRI and are associated with the investigational use of monoclonal antibodies targeting [beta amyloid], including aducanumab, in patients with Alzheimer disease," the group noted.
Two clinical trials, EMERGE and ENGAGE, were conducted between August 2015 and March 2019 and offer data for assessing whether patients who are treated with the drug go on to develop ARIA. The trials compared low-dose aducanumab treatment (i.e., 3 mg/kg to 6 mg/kg), high-dose aducanumab treatment (i.e., 6 mg/kg to 10 mg/kg), and a placebo and included 3,285 participants with Alzheimer's disease. Patients were subcategorized by apolipoprotein E ε4 allele carrier status and the aducanumab dose was determined by this status.
Of the total study participant pool, 1,087 patients received a placebo and 2,198 received aducanumab in either high or low doses. Patients were tracked for occurrence of amyloid-related imaging abnormalities with follow-up MRI exams over a period of 19 months; ARIA manifested as vasogenic edema or effusion, and its severity was characterized by extent of involvement.
Salloway and colleagues sought to describe imaging and clinical characteristics of amyloid-related imaging abnormalities using data from these two trials. Their study found that 41.3% of patients experienced ARIA, exhibited as either ARIA edema (ARIA-E) or ARIA caused by hemosiderin deposits (ARIA-H).
ARIA-E was the most common adverse event (35.2%) resulting from aducanumab treatment, the group noted. The highest occurrence of ARIA-E was in those patients treated with aducanumab who were apolipoprotein E ε4 allele carriers, and the study also found that ARIA events most often occurred early in treatment (within the first eight doses of the drug).
The MRI findings determined whether patients' aducanumab dose would be suspended or discontinued, Salloway and colleagues explained.
"For radiographically mild ARIA, dosing was continued if ARIA was asymptomatic; if symptoms were present, dosing was suspended," they wrote. "Following detection of moderate or severe ARIA-E or detection of moderate ARIA-H, dosing was suspended until resolution of ARIA-E and stabilization of ARIA-H ... On detection of an ARIA episode, follow-up MRIs were conducted approximately every four weeks to document resolution of ARIA-E or stabilization of ARIA-H."
The study results could help clinicians better understand the effects of aducanumab in Alzheimer's patients, according to the team.
"Although ARIA can occur at any time, clinical suspicion for ARIA should be highest in early treatment, and routine surveillance MRIs should be supplemented with ad hoc MRIs in patients with new-onset symptoms potentially associated with ARIA," the group concluded.