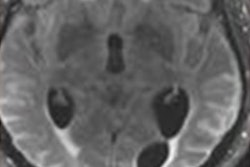
The U.S. Food and Drug Administration (FDA) has updated safety labeling for Biogen's Alzheimer's disease drug aducanumab (Aduhelm) to include two additional MRI scans for patients during the first year of treatment.
On top of two MRI scans already recommended, the additional scans are meant for earlier detection of amyloid-related imaging abnormalities (ARIA), which manifest as brain edemas or sulcal effusions and may be associated with seizures in some patients.
ARIAs comprise a spectrum of imaging findings detected on brain MRI and are associated with the use of monoclonal antibodies like aducanumab that target beta amyloid in patients with Alzheimer disease, according to studies. The label change brings the total number of MRI scans recommended for patients prescribed aducanumab to five, including an initial scan within a year before starting therapy.
Biogen announced the updated label in a news release on April 29, adding that discontinuation of treatment is advised when ARIA are detected, depending on severity.
In addition, based on the company's ongoing pharmacovigilance, the updated labeling for Aduhelm now includes information in the Warnings and Precautions section describing that seizures can be a serious symptom of ARIA. In phase III clinical studies of aducanumab, 0.3% of patients treated with the FDA recommended dose reported serious symptoms associated with ARIA. In these trials, seizure occurred in 0.7% of patients, the company said.
Aducanumab is indicated for patients with mild cognitive impairment who have been diagnosed with Alzheimer's disease based on the presence of beta amyloid, a hallmark of the disease associated with cognitive decline. The drug was approved by the FDA in June 2021 under an accelerated regulatory pathway, and has since faced multiple hurdles.
Most recently, the U.S. Centers for Medicare and Medicaid Services (CMS) finalized a plan April 7 that limits coverage of aducanumab only to patients enrolled in clinical trials.