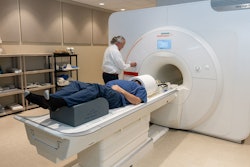
Siemens Healthineers has secured clearance from the U.S. Food and Drug Administration (FDA) for its Magnetom Cima.X 3-tesla MRI whole-body scanner.
At the heart of the Magnetom Cima.X is its Gemini Gradients, which have an amplitude of 200 mT/m and a slew rate of 200 T/m/s., according to the vendor. The gradients are 2.5 times higher than the strongest whole-body MR gradients currently available from Siemens Healthineers, the company said, adding that the high gradient level helps clinicians better study neurodegenerative diseases by increasing the visibility of microstructures that cannot be seen clearly with traditional MR scans.
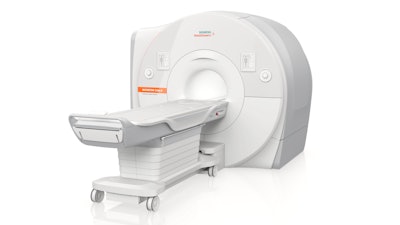 Magnetom Cima.X 3-tesla MRI whole-body scanner. Image courtesy of Siemens Healthineers.
Magnetom Cima.X 3-tesla MRI whole-body scanner. Image courtesy of Siemens Healthineers.
The scanner includes a new physiologging feature for precise, time-stamped physiological data from the patient, and Siemens' Open Recon platform that allows clinicians to run custom algorithms for image reconstructions directly on the scanner, the vendor said. Open Recon also allows for data-sharing between MR scanners and institutions for research.
Siemens said that the scanner also includes the following:
- BioMatrix technology that automatically adjusts for user variability, as well as anatomical and physiological differences of patients to reduce repeated scans
- Deep Resolve reconstruction technology powered by AI to speed up MR scans, by up to 70% in the case of brain scans.
- myExam Companion workflow tool that incorporates AI to help radiologic technologists of varying experience navigate the MR exam


.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=100&q=70&w=100)



.fFmgij6Hin.png?auto=compress%2Cformat&fit=crop&h=167&q=70&w=250)