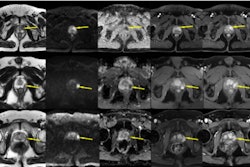
SPL Medical B.V. has announced results from its PROSTAPROGRESS study, which assessed the diagnostic accuracy of ferumoxtran-enhanced MRI for lymph node detection in prostate cancer patients.
Ferumoxtran, an iron-based investigational MRI contrast agent, met its primary endpoints in the phase III study, SPL Medical said in a statement.
The study, which concluded in January, was conducted with prostate cancer patients with a medium to high risk of lymph node metastases and without prior treatment at university centers in Germany, Netherlands, Belgium, and Switzerland.
Safety data from ongoing safety monitoring revealed ferumoxtran had consistent performance compared with other MRI contrast agents. Angiography data from the trial showed mostly excellent or good angiography readability as judged by the central readers, the company added.
Ferumoxtran can be applied in MRI as a safe blood-pool agent for angiography, as well as for functional diagnostics in the detection of even very small lymph node metastases, according to the firm, and the dosage used for it is significantly lower than those of widely-used iron supplementation products.