A PET radiotracer that detects clear-cell renal cell carcinoma proved safe and effective in a clinical trial conducted at 36 research hospitals across nine countries, according to a study published September 10 in the Lancet Oncology.
The imaging agent fills an unmet need to identify small lesions, which could allow earlier diagnosis of the disease, noted lead author Brian Shuch, MD, of the University of California, Los Angeles, and colleagues.
"Imaging using [zirconium-89] girentuximab has the potential to change clinical practice in renal cell carcinoma, including staging and monitoring patients at high risk and detection of distant metastasis," the group wrote.
Patients with small clear-cell renal cell carcinoma (ccRCC) tumors have a five-year survival rate of up to 70%, yet when tumors are already large, treatment is more difficult and the five-year survival rate is about 10%. People with small tumors and without symptoms are typically diagnosed incidentally by imaging exams performed for other reasons, the authors explained.
In previous smaller studies, zirconium-89 (Zr-89) girentuximab (Zircaix, Telix Pharmaceuticals) PET/CT imaging differentiated ccRC tumors from other kidney tumor types based on its ability to bind to carbonic anhydrase 9 (CAIX), an antigen highly expressed by ccRCC cancer cells. In this study, the researchers evaluated the technique in patients with suspicious renal masses (≤ 7 cm in diameter) who were scheduled for nephrectomy.
The coprimary endpoints, determined by three individual readers, were the sensitivity and specificity of Zr-89 girentuximab PET/CT imaging to detect clear-cell renal cell carcinoma, with confirmation by biopsies serving as the standard of truth.
Between August 2019 and July 2022, the group enrolled 284 participants. Patients received a single dose of the tracer intravenously followed by abdominal PET/CT five days (±2 days) later, with surgery performed no later than 90 days after imaging.
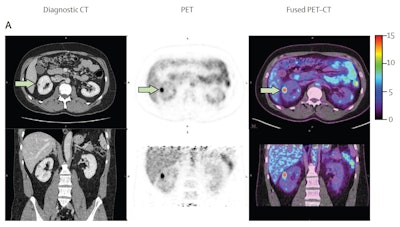 A 40-year-old male with 12 mm lesion in superior right kidney showing positive Zr-89 girentuximab PET and histologically confirmed clear-cell renal cell carcinoma.Images available under Creative Commons license (CC BY 4.0 DEED, Attribution 4.0 International) and courtesy of the Lancet Oncology.
A 40-year-old male with 12 mm lesion in superior right kidney showing positive Zr-89 girentuximab PET and histologically confirmed clear-cell renal cell carcinoma.Images available under Creative Commons license (CC BY 4.0 DEED, Attribution 4.0 International) and courtesy of the Lancet Oncology.
Based on the expert reads, the mean sensitivity for identifying ccRCC on the Zr-89 girentuximab PET/CT images for all patients was 85.5% and the mean specificity was 87%, according to the results. Notably, in a subgroup analysis of 20 patients with indeterminate renal masses ≤2 cm, mean sensitivity and specificity was 96.7%.
Most adverse events were not or were unlikely to be related to Zr-89 girentuximab, with most (193 [74%] of 261 events) occurring during or after surgery, the authors added.
Ultimately, the incidence of small renal masses is increasing, according to the authors. Diagnoses and treatments are limited by current imaging techniques and many patients undergo unnecessary surgery to remove masses that are later determined to be benign, they noted.
“These results establish the value of Zr-89 girentuximab PET/CT imaging as a new standard, non-invasive tool for the diagnosis and detection, characterization, and differentiation of clear-cell renal cell carcinoma from other renal and extrarenal lesions in clinical practice, minimizing the risk of unnecessary invasive interventions,” the group concluded.
The full study is available here.