The American Brachytherapy Society (ABS) and the American Society of Breast Disease (ASBD) have issued statements identifying what they believe are deficiencies and misleading information in a study on breast brachytherapy published in the May 2 issue of the Journal of the American Medical Association.
The study, undertaken by researchers from MD Anderson Cancer Center, used Medicare claims data to compare survival outcomes and toxicities of almost 93,000 women who underwent accelerated partial-breast irradiation (APBI) brachytherapy or whole-breast irradiation following a lumpectomy for early-stage breast cancer.
This is the second round of comments by ABS and ASBD, which also issued statements when the study results were first presented in December 2011 at the San Antonio Breast Cancer Symposium (SABCS).
In its most current statement, ABS pointed out that the paper published in JAMA "tells us very little in contrast to the results of many carefully performed studies of APBI-brachytherapy accumulated over 20 years." The organization also noted that "the method of analysis in which two groups missing vital information are compared is crude at best, and carries the inherent risk of leading to tenuous or false conclusions."
The ABS also restated its concern regarding the potential impact of the study on patients. "Statements that disparage the use of breast brachytherapy based on the data presented in this study are therefore without merit and irresponsible," and false biases could be developed that might deprive eligible patients of the treatment.
For its part, ASBD reiterated its criticisms of what it called study flaws and deficiencies that were made by both breast surgeons and radiation oncologists following the initial presentation of the paper at SABCS, and again last week in JAMA.
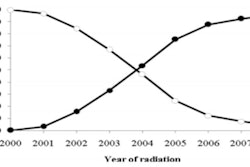
This time it also emphasized that the analysis focused on a time period when surgeons, radiation oncologists, and medical physicists were less experienced and "just beginning the learning curve" in the use of APBI. It also pointed out that a more sophisticated generation of APBI applicators were introduced in 2006. For this reason, the MD Anderson study was using historic data rather than contemporary data and could mislead physicians and their patients, ASBD said.
The ABS statement can be read by clicking here and the ASBD statement by clicking here.