Sonazoid, an ultrasound contrast agent that differentiates normal from abnormal organ tissue, passed preliminary muster both for detecting liver lesions and enhancing the prostate, say two studies presented at the 2001 American Institute of Ultrasound in Medicine meeting in Orlando.
The agent is one of a new breed of contrast agents in which echogenic microbubbles are injected into the bloodstream. It is manufactured by Buckinghamshire, UK-based Nycomed Amersham and is not yet approved for marketing in the U.S.
Sonazoid’s tiny bubbles are derived from a lipid-stabilized suspension of perfluorocarbon. The agent is injected, and the bubbles course through the bloodstream until they’re absorbed by healthy liver and prostate cells. Next, ultrasound waves rupture the bubbles, and the resulting signal is easily detected by sonography. The lack of a rupture signal in a given area indicates a lack of healthy cells, meaning the likely presence of cancer.
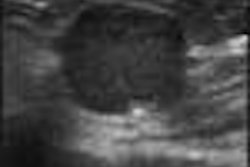
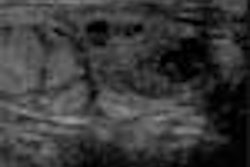
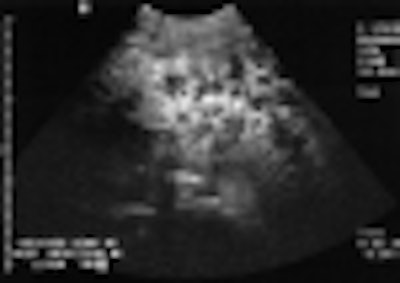
When used to detect liver lesions, for example, Sonazoid is absorbed by normal Kupffer cells. Any surrounding tumor tissue, which displaces the Kupffer cells, appears unenhanced on ultrasound scans and is therefore easily detected.
In order to test the clinical effectiveness of this phenomenon, researchers developed a multinational, four-center study in which 52 patients with known focal liver lesions were first imaged with unenhanced ultrasound.
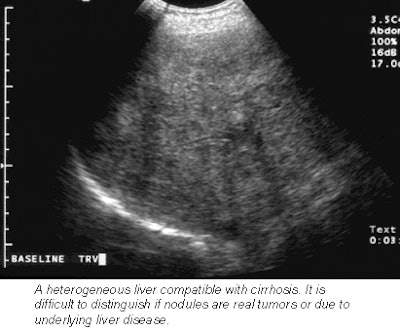 |
Next, each patient received an injection of Sonazoid, which was immediately followed by a pulse-inversion harmonic ultrasound scan that tracked the contrast in the bloodstream. A second scan 15 minutes later detects the contrast as it accumulates in liver cells. The contrast-enhanced scans boasted a 76% improvement in lesion conspicuity, a sensitivity improvement from 73% to 89%, and a jump in the number of confirmed metastases from 68 to 83.
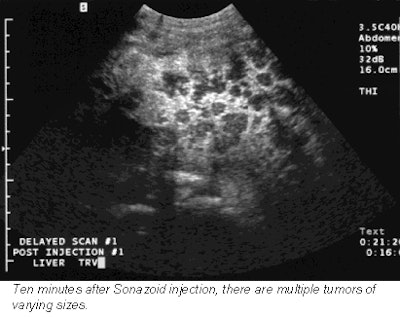 |
These promising results can be credited to Sonazoid’s long-lasting parenchymal phase, which is a measure of how long the bubbles remain in healthy liver cells before dissipating, said Dr. Laurence Needleman, an associate professor of radiology at Thomas Jefferson University in Philadelphia.
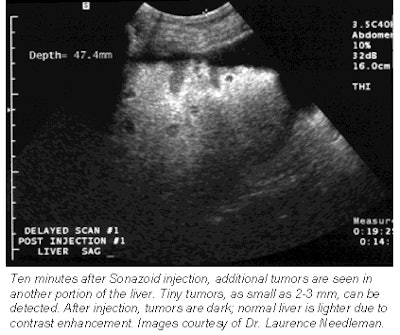 |
"Because the agent stays in the cells for many minutes, even up to one hour after the delivery of the agent, the scans are more specific," Needleman said. "And you’re not under time pressure to perform the scan as soon as you’ve injected the agent, which makes it logistically easy."
Needleman noted, however, that a small number of benign lesions also absorbed the contrast, meaning these lesions may resemble healthy tissue on ultrasound. This potential ambiguity is mitigated by the fact that many benign masses absorb the contrast more slowly than healthy tissue, a telltale difference that can be sonographically detected. In addition, some benign lesions show a robust and thus suspicious degree of vascularity that can be detected in the first scan, taken when the contrast is in the bloodstream.
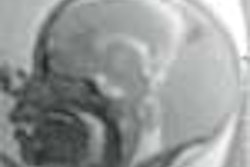
Sonazoid also shows promise in the prostate. A smaller study conducted by a different team at Thomas Jefferson University determined that Sonazoid demonstrated diffuse gray-scale enhancement in the transition zone between the inner and outer layers of the prostate.
The investigators evaluated nine prostate cancer patients with unenhanced gray-scale and wide-band harmonic imaging, and then performed identical scans after infusing Sonazoid. All results were subsequently compared with findings obtained after radical prostatectomy. In addition to enhancing the transition area, Sonazoid demonstrated increased blood flow to the outer portion of the gland. However, the authors reported that only half of the tumor foci were detected via contrast enhancement.
Both of these studies are preliminary, and more work is needed to prove Sonazoid's clinical merits. In liver cancer, for example, the next step is to test the agent's specificity by administering it to patients who may or may not have lesions, unlike Needleman's recent study in which all patients had lesions as detected by MRI or CT, he said.
But even if the agent earns its clinical stripes, Needleman cautions that it may not get FDA approval. The agency places as much emphasis on whether a contrast agent improves patient outcomes as to whether it improves cancer detection -- a tall order for an agent that targets both liver and prostate cancer. These are two of the leading causes of cancer death in the U.S., he added.
"Sonazoid would certainly give people information they need to know, but whether that translates into an increased lifespan is another question," he said. "For now it may not, because cancer diagnosis is outpacing treatment."
Nycomed Amersham is also examining the use of Sonazoid for imaging myocardial perfusion, or the flow of blood to the heart. And Sonazoid isn’t the only microbubble-based contrast product working its way through clinical trials. Berlin-based Schering AG has developed Levovist, a galactose-based agent that is already approved in Japan and Europe, and is undergoing phase-III trials in the U.S. The company has also developed Sonovist, a cyanoacrylate polymer-based agent, which is undergoing phase-II trials.
By Dan Krotz
AuntMinnie.com contributing writer
May 21, 2001
Related Reading
Nycomed and Mallinckrodt settle patent suits, May 8, 2000
Click here to post your comments about this story. Please include the headline of the article in your message.
Copyright © 2001 AuntMinnie.com